-
PDF
- Split View
-
Views
-
Cite
Cite
Tamás Székely, Innes C. Cuthill, János Kis, Brood desertion in Kentish plover: sex differences in remating opportunities, Behavioral Ecology, Volume 10, Issue 2, March 1999, Pages 185–190, https://doi.org/10.1093/beheco/10.2.185
Close - Share Icon Share
Abstract
To understand the evolution of parental care, one needs to estimate the payoffs from providing care for the offspring and the payoffs from terminating care and deserting them. These payoffs are rarely known. In this study we experimentally estimated the rewards from brood desertion in a species that has a variable pattern of parental care. In particular, either the female or the male parent may desert the brood in Kentish plover Charadrius alexandrinus, so some broods are attended by one parent of either sex, whereas in other broods both parents stay with the brood until the chicks fledge. We created single males and single females by experimentally removing the other parent and the clutch. The expected remating time of males was significantly higher (median: 25.4 days) than that of the females (5.3 days, p <.0001). The expected remating time tended to increase over the breeding season in both sexes, although the increase was significant only in females. The new nest of remated males was closer to their previous territory (mean ± SE, 46 ± 8 m) than that of the remated females (289± 57 m, p <.001). Hatching success of new nests was not different between remated males and females. Our results demonstrate that the remating opportunities are different for male and female Kentish plovers and these opportunities vary over the season. We propose that the remating opportunities were influenced by the male-biased adult sex ratio and the seasonal decrease in the number of breeders. However, we stress that measuring remating times is a more direct measure of mating opportunities than calculating the operational sex ratio.
The evolution of parental care is often explained by the relative magnitude of two benefits (Carlisle, 1982; Lazarus, 1990; Maynard-Smith, 1977). One benefit is derived from the reproductive value of the offspring and is affected by the provision of care. Thus, if parents improve the survival chances of their offspring (e.g., by feeding them or protecting them from predators), then parents may increase their reproductive success by caring for their offspring until their young became fully independent. This benefit should be traded off against the benefit of deserting the offspring. If desertion confers advantages over staying with the brood such as by allowing an extra reproductive bout for the parent or by enhancing the parents' prospects for survival until future breeding seasons, then parents may desert before their offspring are independent (reviewed by Clutton-Brock, 1991; Székely et al., 1996).
The payoffs for caring and for deserting may be different for the two sexes. First, the value of care may differ between males and females. For example, in many passerines only females have brood patches, so incubation by the female is more valuable than incubation by the male. Second, males and females may have different payoffs from deserting. For example, if the operational sex ratio (OSR; Emlen and Oring, 1977), the ratio of available females to available males, is biased, then one sex has a higher chance of remating and reproducing than the other. The payoff from deserting may also vary over the breeding season. For example, the OSR may fluctuate because of different arrival times of males and females to the breeding ground. Also, if breeding is seasonal, then as the end of the season approaches, the chance of successfully completing a new breeding attempt should diminish.
Although theoretical studies have pointed out that understanding these payoffs is crucial for revealing why parents care for their offspring (Balshine-Earn and Earn, 1997; Lazarus, 1990; Maynard Smith, 1977), empirical studies are scarce (reviewed by Clutton-Brock, 1991; Balshine-Earn, 1995; Székely et al., 1996). Simple observation of the behavior of parents (whether they care or desert) and estimates of their subsequent reproductive success are not sufficient because such behavior may be confounded by the individual qualities of parents. For example, those parents that provide better than average quality of care for their offspring may be more willing to stay with their offspring, and offspring of such parents may have a better than average chance of fledging. Furthermore, these payoffs should be obtained from a species in which offspring desertion is part of a natural breeding strategy (Clutton-Brock, 1991). Finally, these payoffs should be estimated in the environment where the different patterns of parental care emerge naturally.
We investigated one such appropriate species, the Kentish plover Charadrius alexandrinus. The Kentish plover has a variable parental care pattern—after the eggs hatch, either the male or the female parent often deserts the brood (Fraga and Amat, 1996; Lessells, 1984; Paton, 1995; Warriner et al., 1986). Their mating pattern is also variable; both sequential polygyny and polyandry occur because after desertion males and females may remate and renest. At least 37% and 27% of deserting females remated in California and in Hungary, respectively (Székely and Williams, 1995; Warriner et al., 1986). Therefore, a major benefit of desertion appears to be reproduction in the year of desertion. The aim of our study was to quantify the remating opportunity and the reproductive success of a deserting parent. In particular, we investigated whether the benefit of desertion is different for males and females and whether this benefit varies over the breeding season. In a companion study we analyze the payoffs from provision of care (Székely and Cuthill, this issue).
METHODS
Experimental manipulation
Field work was carried out at Tuzla Lake (36°43′ N, 35°03′ E), 2 km south of Tuzla village in the Çukurova Delta, southern Turkey. Approximately 1000 pairs of Kentish plovers breed there. The experiment was carried out on the north side of the lake in an area of approximately 1.5 km2. This site was distinct from site B used in our companion experiment on the value of care (Székely and Cuthill, this issue) and overlapped with site A, although a bird, nest, or brood was involved only in one of the two experiments. The study site was a saltmarsh bordered by arable land to the north and the lake to the south. Halophytes such as Salicornia europaea and Sueda prostrata were the most common plants (Uzun et al., 1995).
Both parents were caught on their nest between 16 April and 16 June 1996 (n = 40 pairs). Body mass and tarsus length of each parent were measured, and birds were ringed with an individual combination of color rings. We scored the amount of intrafurcular fat using the method of Helms and Drury (1960). One parent was released in each pair (released-1), whereas the other parent was taken into captivity. For each pair of nests we randomized whether the male or the female parent was released. Before a parent was released, its clutch was taken away and the size of the eggs was measured. We estimated the number of days that these eggs had been incubated by floating the eggs in lukewarm water (Kis J and Székely T, unpublished data). Only nests with three eggs (modal clutch size) were manipulated. The eggs were distributed into other nests that were not included in this study. The experiment was licensed by the Turkish Ministry for Natural Parks in a location where Kentish plovers are abundant (Magnin and Yarar, 1997).
After the released-1 plovers remated or had not been seen at the study site for at least 1 week, their previous mate was released from captivity (released-2). We also investigated the remating behavior of these plovers. One female, which was released from captivity 7 days after her mate was not seen on his territory, found her previous mate. This pair was excluded from all analyses of remated birds.
We searched for released plovers every day until 30 June 1996. The searched area extended farther east and west than the core where the manipulations took place, and we regularly covered approximately 3.5 km2. Once a bird was found, we recorded its behavior and identified whether the bird had remated. A bird was considered remated if the courtship of a male was accepted by a female; that is, a female followed a male and they prepared a nest-scrape or copulated. In 1 out of 28 remated plovers the pair-bond was not permanent, giving 27 remated birds. In addition, seven plovers were not observed to remate, although we found their new nest. The maximum remating time was assumed for these plovers (i.e., we assumed that they found a mate the day before the first egg had been laid for the new nest). New clutches were checked at least every other day and daily around the expected date of hatching. Successful clutches hatched at least one chick. Three females were observed only once after 22, 37, and 52 days of release, when these females appeared to be single. These observations were excluded from the analyses of remating times, as they were more than 4 SDs away from the mean of the female population.
We aimed at recording the behavior of parents three times each before they remated and after they remated. No data were collected after the new nest of remated plovers was completed. During a behavioral sample, we scanned the behavior of the focal plover and its mate (if mated) every 20 s for 30 min. A variety of behaviors were recorded. Here we focus on those that relate to display behavior (courtship, scraping nestscrape, and fighting with other Kentish plovers) and self-maintenance (pecking at a prey item and preening). The distance between the focal parent and its previous nest (“distance from territory”) was estimated every 5 mins.
To facilitate the recognition of experimental birds in the field, we dyed the flanks of released birds with picric acid. This was necessary to identify the experimental birds in the large nonexperimental population. To check whether the dye influenced mating behavior, two males and three females were left undyed. Mating behavior of dyed and undyed birds was not different: two undyed males stayed single for 15 and 22 days (range of dyed males: 1-52 days, n = 28 males), and an undyed female stayed single for 3 days (range of dyed females: 0-13 days, n = 22 females).
Data processing and statistical analyses
We calculated volumes of completed clutches using a specific egg-shape index for the Kentish plover (Székely et al., 1994). Body mass was related to the intrafurcular fat score at the time of manipulation in females (Pearson correlation, r =.324, p =.041, n = 40), but not in males (r = -.116, p =.482, n = 39). Behavioral variables were arcsine square-root transformed, and distance data were log10 (x + 1) transformed. Behavioral units were the proportion of time out of all observations when the focal bird was in view. Behavior of released-1 and released-2 plovers was not different (two-way MANOVA, type of release: Wilk'sλ = 0.929, p =.793; sex: λ = 0.672, p =.025; interaction: λ = 0.732, p =.072). Because some of the behavioral variables violated the assumptions of parametric tests, we also investigated them by nonparametric statistics. Nonsignificant interaction terms (p >.05) in two-way ANOVAs and MANOVAs are not reported.
Date of remating was defined as the mean date between the date a plover was seen single and the first date it was with a new mate. Remating time was the difference between date of release and date of remating. Remating times were 1n(x + 1) transformed and analyzed by parametric tests. These analyses, however, did not take into account birds that did not remate. Therefore, we also calculated the remating time of the sexes using survival analyses (Norušis, 1994) and refer to these estimates as expected remating times. In the latter analyses the terminal event (outcome) was the occurrence of remating defined as the first observation when a plover was seen with a new mate. Several individuals did not find a new mate when we saw them for the last time, and we treated these observations as censored (Norušis, 1994). The proportion of censored observations did not differ between males (13 out of 32) and females (12 out of 27, χ2 test with continuity correction, χ2 = 0.001, p =.975). D (which follows a χ2 distribution) and probability of Lee-Desu tests are given for the survival analyses (Norušis, 1994). Median remating time is the time when 50% of single birds are expected to pair. We used Cox regression to investigate the effect of date of release on remating time (Norušis, 1994). For the latter analyses we give B of the cumulative survival function and its significance (Norušis, 1994). Note that when B is negative, then remating times increase over the season. The effects of date of release (covariate) and sex of parent (factor) on remating time was investigated by a Cox regression model in which we also included the interaction between the factor and the covariate. Slopes of two or more regression equations were compared by full models of ANOVAs in which remating time was the dependent variable, sex was the factor, and tarsus length and body condition, or the amount of intrafurcular fat were the independent variables. For these ANOVAs the significance of the interaction terms between the factor and the independent variables are given. Dates are given as number of days since 1 March. Two-tailed probabilities and means ± SEs are given unless otherwise indicated. We used SPSS for the Macintosh 4.0, SPSS for Windows 6.1, and MINITAB (1995) 10.1 for Windows for data processing.
RESULTS
At manipulation
We found no difference between male-released and female-released pairs in volume and in laying date of removed clutches (t tests, p =.310 and.762, respectively), nor in the number of days they had been incubating their clutch (t test, p =.276). Furthermore, male-released and female-released pairs were not different in body mass (two-way ANOVAs, male-released versus female-released: F1,76 = 0.896, p =.347, sex: F1,76 = 0.061, p =.805), fat reserves (male-released versus female-released: F1,75 = 0.519, p =.473, sex: F1,75 = 1.999, p =.161), or in tarsus length (male-released versus female-released: F1,76 = 0.985, p =.324, sex: F1,76 = 28.260, p <.001). Finally, neither body mass nor fat reserves of males and females were different in released-1 (t tests, p =.653 and.143, respectively) and in released-2 birds (p =.779 and.736, respectively), but males were larger than females in terms of their tarsus length (t tests, released-1: t = 3.29, p =.002, released-2: t = 3.52, p =.002).
Release from captivity
Thirteen males and 10 females were released from captivity before or on 16 June. Males and females were released from captivity 79.6 ± 4.7 days and 87.6 ± 3.9 days after 1 March, respectively (t test, t = 1.25, p =.225). Males spent less time in captivity (13.3± 1.9 days) than females (23.8 ± 4.2 days, t = 2.27, p =.041) because their previous mate paired more quickly than the previous mates of females (see below).
Behavior of males and females
The transition from single to remated did not significantly influence the behavior of males and females, but the behavior of sexes was clearly different (two-way MANOVA, remated status: λ = 0.776, p =.144; sex:λ = 0.534, p <.001). In particular, males spent more time courting (two-way ANOVAs with sex and remated status, sex: F1,35 = 16.032, p <.001), scraping (F1,35 = 9.981, p =.003), fighting (F1,35 = 5.947, p =.020), and preening (F1,35 = 5.819, p =.021) than females. On the other hand, females tended to spend more time feeding than males (F1,35 = 4.093, p =.051, Table 1). The behavior of males and females was unrelated to the time in the breeding season (Pearson correlations, males: r = -.353-.064, p =.077-0.927, n = 26, females: r = -.421-.256, p =.152-.871, n = 13).
Behavior of single and remated plovers
| . | Mean ± SE % of time . | Za . | p . | |
|---|---|---|---|---|
| . | Male . | Female . | . | . |
| aZ value and probability from Mann-Whitney U test. | ||||
| Single | ||||
| Courting | 3.65 ± 1.03 | 0.23 ± 0.23 | 2.029 | .043 |
| Scraping | 2.44 ± 1.03 | 0.00 ± 0.00 | 1.694 | .090 |
| Fighting | 3.38 ± 1.80 | 0.12 ± 0.12 | 1.785 | .074 |
| Feeding | 8.71 ± 1.73 | 9.91 ± 3.07 | 0.462 | .644 |
| Preening | 5.44 ± 1.23 | 1.28 ± 0.65 | 1.898 | .058 |
| No. of birds | 19 | 5 | ||
| Remated | ||||
| Courting | 6.03 ± 2.62 | 0.14 ± 0.14 | 2.950 | .003 |
| Scraping | 11.29 ± 4.59 | 0.95 ± 0.85 | 2.008 | .045 |
| Fighting | 1.40 ± 0.33 | 0.07 ± 0.07 | 2.950 | .003 |
| Feeding | 2.18 ± 0.71 | 9.02 ± 2.43 | 2.323 | .020 |
| Preening | 6.71 ± 2.15 | 1.81 ± 0.64 | 1.532 | .126 |
| No. of birds | 7 | 8 | ||
| . | Mean ± SE % of time . | Za . | p . | |
|---|---|---|---|---|
| . | Male . | Female . | . | . |
| aZ value and probability from Mann-Whitney U test. | ||||
| Single | ||||
| Courting | 3.65 ± 1.03 | 0.23 ± 0.23 | 2.029 | .043 |
| Scraping | 2.44 ± 1.03 | 0.00 ± 0.00 | 1.694 | .090 |
| Fighting | 3.38 ± 1.80 | 0.12 ± 0.12 | 1.785 | .074 |
| Feeding | 8.71 ± 1.73 | 9.91 ± 3.07 | 0.462 | .644 |
| Preening | 5.44 ± 1.23 | 1.28 ± 0.65 | 1.898 | .058 |
| No. of birds | 19 | 5 | ||
| Remated | ||||
| Courting | 6.03 ± 2.62 | 0.14 ± 0.14 | 2.950 | .003 |
| Scraping | 11.29 ± 4.59 | 0.95 ± 0.85 | 2.008 | .045 |
| Fighting | 1.40 ± 0.33 | 0.07 ± 0.07 | 2.950 | .003 |
| Feeding | 2.18 ± 0.71 | 9.02 ± 2.43 | 2.323 | .020 |
| Preening | 6.71 ± 2.15 | 1.81 ± 0.64 | 1.532 | .126 |
| No. of birds | 7 | 8 | ||
Behavior of single and remated plovers
| . | Mean ± SE % of time . | Za . | p . | |
|---|---|---|---|---|
| . | Male . | Female . | . | . |
| aZ value and probability from Mann-Whitney U test. | ||||
| Single | ||||
| Courting | 3.65 ± 1.03 | 0.23 ± 0.23 | 2.029 | .043 |
| Scraping | 2.44 ± 1.03 | 0.00 ± 0.00 | 1.694 | .090 |
| Fighting | 3.38 ± 1.80 | 0.12 ± 0.12 | 1.785 | .074 |
| Feeding | 8.71 ± 1.73 | 9.91 ± 3.07 | 0.462 | .644 |
| Preening | 5.44 ± 1.23 | 1.28 ± 0.65 | 1.898 | .058 |
| No. of birds | 19 | 5 | ||
| Remated | ||||
| Courting | 6.03 ± 2.62 | 0.14 ± 0.14 | 2.950 | .003 |
| Scraping | 11.29 ± 4.59 | 0.95 ± 0.85 | 2.008 | .045 |
| Fighting | 1.40 ± 0.33 | 0.07 ± 0.07 | 2.950 | .003 |
| Feeding | 2.18 ± 0.71 | 9.02 ± 2.43 | 2.323 | .020 |
| Preening | 6.71 ± 2.15 | 1.81 ± 0.64 | 1.532 | .126 |
| No. of birds | 7 | 8 | ||
| . | Mean ± SE % of time . | Za . | p . | |
|---|---|---|---|---|
| . | Male . | Female . | . | . |
| aZ value and probability from Mann-Whitney U test. | ||||
| Single | ||||
| Courting | 3.65 ± 1.03 | 0.23 ± 0.23 | 2.029 | .043 |
| Scraping | 2.44 ± 1.03 | 0.00 ± 0.00 | 1.694 | .090 |
| Fighting | 3.38 ± 1.80 | 0.12 ± 0.12 | 1.785 | .074 |
| Feeding | 8.71 ± 1.73 | 9.91 ± 3.07 | 0.462 | .644 |
| Preening | 5.44 ± 1.23 | 1.28 ± 0.65 | 1.898 | .058 |
| No. of birds | 19 | 5 | ||
| Remated | ||||
| Courting | 6.03 ± 2.62 | 0.14 ± 0.14 | 2.950 | .003 |
| Scraping | 11.29 ± 4.59 | 0.95 ± 0.85 | 2.008 | .045 |
| Fighting | 1.40 ± 0.33 | 0.07 ± 0.07 | 2.950 | .003 |
| Feeding | 2.18 ± 0.71 | 9.02 ± 2.43 | 2.323 | .020 |
| Preening | 6.71 ± 2.15 | 1.81 ± 0.64 | 1.532 | .126 |
| No. of birds | 7 | 8 | ||
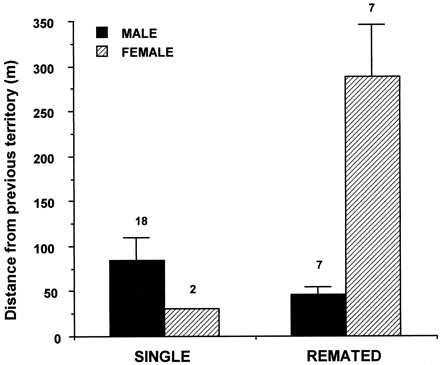
Single males and females were observed at similar distances from their previous territory (Figure 1; Mann-Whitney U test, Z = 1.512, p =.131), whereas remated males stayed nearer to their previous territory than remated females (Figure 1; t test, t = 5.99, p <.001). A significant interaction between sex and remated status indicated that females moved to the territory of their new mate, whereas new mates of remated males moved to the territory of their males (two-way ANOVA, sex: F1,30 = 8.836, p =.006; mated status: F1,30 = 1.429, p =.241; interaction: F1,30 = 10.737, p =.003).
Distance of single and remated plovers from their previous territory (means± SE). The number of individuals is shown above each bar.
Time to remate
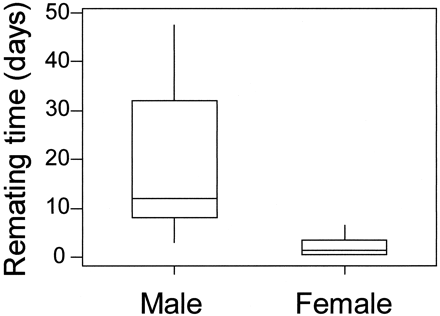
Females remated more quickly (median = 1.5 days, range: 0.5-6.5 days, n = 15) than males (median = 12.0 days, range: 3.0-47.5 days, n = 19, t = 7.39, p <.001; Figure 2). Remating times remained different between males and females after excluding six males and one female for which remating times were estimated (see Methods; t = 6.25, p <.001). Captivity did not influence remating times, as released-2 plovers took as much time to remate as released-1 plovers (two-way ANOVA, release type: F1,30 = 0.776, p =.385; sex: F1,30 = 53.973, p <.001).
Box-plots of remating time of male and female Kentish plovers. The line is drawn across the median, the bottom and the top of the box are lower (Q1) and upper quartiles (Q3), respectively. The whiskers extend from the lower and the upper quartiles to the lowest and highest observation, respectively, within the range defined by Q1 - 1.5*(Q3 - Q1) and Q3 + 1.5*(Q3 - Q1) (MINITAB, 1995).
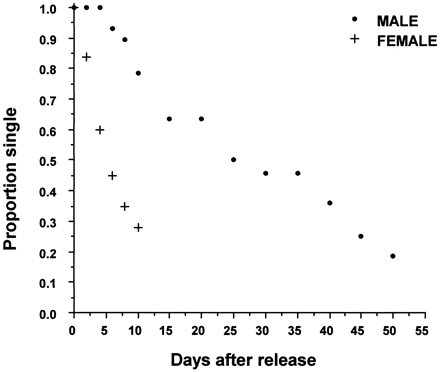
These analyses, however, do not take into account that several plovers remained single when they were observed for the last time in the season. By using survival analyses we found that the expected remating times of females (median = 5.3 days, n = 27 females) remained significantly less than that of the males (median = 25.4 days, n = 32 males, D = 15.382, p <.0001; Figure 3). The expected remating times tended to increase over the season in both sexes, although it was significant only in females (Cox regression, B = -0.028, n = 23 females, p =.044), not in males (B = -0.017, n = 29 males, p =.343). Thus the expected remating times followed the same trend in both sexes (Cox regression, interaction between sex and date of release: B = -0.007, n = 52, p =.543).
Proportion of Kentish plovers remaining single. Plovers were released on day 0.
Remating times were unrelated to body size (as measured by tarsus length) and body condition, both in males (multiple regression with the remating time as dependent variable, tarsus: b = 4.492, p =.587; condition: b = 0.391, p =.874, n = 19) and in females (tarsus: b = -1.380, p =.879; condition: b = 1.004, p =.489, n = 15). The slopes of regression equations were not different between males and females (ANCOVA, tarsus and sex interaction: F1,28 = 0.19, p =.663; condition and sex interaction: F3,28 = 0.05, p =.828). Finally, remating times were unrelated to intrafurcular fat scores both in males (b = 0.095, p =.719, n = 18) and in females (b = -0.247, p =.169, n = 15), and the slopes of regression equations were not different between males and females (ANCOVA, fat and sex interaction: F1,29 = 1.18, p =.285).
Hatching success of new nests
Females completed their new clutch sooner from the time of release than new female mates of males (Table 2). However, clutch size, volume of clutch, and hatching success were not different between released females and new mates of released males (Table 2). The number of chicks produced by a clutch was unrelated to date of egg laying (Spearman rank correlation, males: rs = 0.176, p =.566, n = 13; females: rs = -.181, p =.617, n = 10).
Hatching success of remated plovers with their new mate
| . | Mean ± SE . | t/Z . | p . | |
|---|---|---|---|---|
| . | Male . | Female . | . | . |
| at test and associated probability. | ||||
| bZ value and probability from Mann-Whitney U test. | ||||
| cFisher's Exact test. | ||||
| Clutch | ||||
| No. of clutches | 13 | 10 | ||
| Completion (days) | 22.9 ± 2.5 | 13.9 ± 2.0 | 2.65a | .015 |
| Size | 2.9 ± 0.1 | 2.8 ± 0.1 | 0.85b | .396 |
| Volume (cm3) | 23.9 ± 0.7 | 23.3 ± 1.3 | 0.42a | .682 |
| No. of chicks hatched/nest | 1.5 ± 0.4 | 0.7 ± 0.4 | 1.46b | .146 |
| No. of successful/total clutches | 8/13 | 3/10 | .214c | |
| . | Mean ± SE . | t/Z . | p . | |
|---|---|---|---|---|
| . | Male . | Female . | . | . |
| at test and associated probability. | ||||
| bZ value and probability from Mann-Whitney U test. | ||||
| cFisher's Exact test. | ||||
| Clutch | ||||
| No. of clutches | 13 | 10 | ||
| Completion (days) | 22.9 ± 2.5 | 13.9 ± 2.0 | 2.65a | .015 |
| Size | 2.9 ± 0.1 | 2.8 ± 0.1 | 0.85b | .396 |
| Volume (cm3) | 23.9 ± 0.7 | 23.3 ± 1.3 | 0.42a | .682 |
| No. of chicks hatched/nest | 1.5 ± 0.4 | 0.7 ± 0.4 | 1.46b | .146 |
| No. of successful/total clutches | 8/13 | 3/10 | .214c | |
Hatching success of remated plovers with their new mate
| . | Mean ± SE . | t/Z . | p . | |
|---|---|---|---|---|
| . | Male . | Female . | . | . |
| at test and associated probability. | ||||
| bZ value and probability from Mann-Whitney U test. | ||||
| cFisher's Exact test. | ||||
| Clutch | ||||
| No. of clutches | 13 | 10 | ||
| Completion (days) | 22.9 ± 2.5 | 13.9 ± 2.0 | 2.65a | .015 |
| Size | 2.9 ± 0.1 | 2.8 ± 0.1 | 0.85b | .396 |
| Volume (cm3) | 23.9 ± 0.7 | 23.3 ± 1.3 | 0.42a | .682 |
| No. of chicks hatched/nest | 1.5 ± 0.4 | 0.7 ± 0.4 | 1.46b | .146 |
| No. of successful/total clutches | 8/13 | 3/10 | .214c | |
| . | Mean ± SE . | t/Z . | p . | |
|---|---|---|---|---|
| . | Male . | Female . | . | . |
| at test and associated probability. | ||||
| bZ value and probability from Mann-Whitney U test. | ||||
| cFisher's Exact test. | ||||
| Clutch | ||||
| No. of clutches | 13 | 10 | ||
| Completion (days) | 22.9 ± 2.5 | 13.9 ± 2.0 | 2.65a | .015 |
| Size | 2.9 ± 0.1 | 2.8 ± 0.1 | 0.85b | .396 |
| Volume (cm3) | 23.9 ± 0.7 | 23.3 ± 1.3 | 0.42a | .682 |
| No. of chicks hatched/nest | 1.5 ± 0.4 | 0.7 ± 0.4 | 1.46b | .146 |
| No. of successful/total clutches | 8/13 | 3/10 | .214c | |
DISCUSSION
Sex differences in mating opportunities
Mating opportunities were different for male and female Kentish plovers, as females took less time to find a new mate and complete a new clutch than males. These results are consistent with previous experiments in Kentish plovers which showed that males took more time to find a new mate than females (Székely, 1996). Experiments have also shown that single males took longer to complete a new clutch than a pair would normally need to replace a failed clutch, whereas single females took no longer than pairs (Lessells, 1983). The remating time of experimentally induced single female Kentish plovers was similar across two populations (Portugal: 2.5 ± 0.4 days, Székely, 1996; Turkey: 2.3 ± 0.5 days, Mann-Whitney U test, Z = 0.713, p =.476), and it is consistent with the remating time of naturally deserting females (Hungary: 2.0 ± 0.6 days; Székely and Williams, 1995). Thus females do not seem to have difficulty in pairing. However, male remating times appear to be different among populations because males took less time to remate in Portugal (7.5 ± 1.4 days) than in Turkey (19.7 ± 3.3 days, Mann-Whitney U test, Z = 1.974, p =.048). One explanation for the observed difference in male remating times may be related to population density. In Portugal the breeding density is higher (5-16 nests/ha; Székely and Williams, 1995) than in Turkey (2-6 nests/ha; Székely T, unpublished data), so the number of females that are willing to breed may be higher too. Future studies on remating opportunities and population densities will be helpful to investigate the generality of the presumed relation between remating times of males and breeding density.
The OSR appears to be biased toward males in all populations of Kentish plovers, and there may be two reasons for this putative bias. First, the adult sex ratio may be skewed; that is, there are more males than females capable of reproducing in the population. This explanation is in line with the findings of P. E. Jönsson (personal communication) that there were consistently more males than females in a small breeding population of Kentish plovers during a 10-year study in Sweden. Also, Warriner et al. (1986) estimated that in their population of snowy plovers (C. alexandrinus nivosus) the ratio of adult males to females was 1.4: 1. There may be several explanations for the skewed sex ratio (different primary and secondary sex ratios, mortalities, ages at maturation, or migration schedules between sexes), although the exact reason has not been deduced. Second, more females may be unavailable for breeding than males because they are incubating or attending their young, for example. The latter explanation, however, is unlikely, because both sexes incubate in Kentish plover, and after the chicks hatch, the female parent deserts the young more often than the male.
Given the large difference in mating time between the sexes, it is surprising that some males take the risk of deserting their brood and attempting to attract a new mate ((Fraga and Amat, 1996; Székely and Lessells, 1993). Brood desertion by males often occurs early in the season Székely and Lessells, 1993), which suggests that these males try to breed. The large variation in male remating times found in our study suggests that some males are apparently better at remating than others. These males may be more attractive to females or better at fighting for a mate and territory than other males. Experimental manipulations of males and their territories are required to separate these explanations.
We also found that remating time tended to increase over the breeding season in both sexes, and we suggest two explanations for this trend. First, if the abundance of food declines during the breeding season (Székely and Cuthill, this issue), then deserting parents have to spend more time reaching a condition in which they can initiate breeding. Second, the number of single plovers that are ready to breed may decrease over the season, so parents have to spend more time searching for a new mate near the end of the season. This may be because the proportion of birds willing to breed decreases as the value of reproduction (i.e., fledging success of the young) decreases toward the end of the season (Székely and Cuthill, this issue). The duration of the breeding season may influence the observed mating patterns. For instance, in many Arctic birds the breeding season is short, which allows only one breeding attempt (Gratto-Trevor, 1991). However, when a breeding season was exceptionally long, female dunlins Calidris alpina had time to mate with new males and renest (Soikkeli, 1967).
Benefits of desertion
Deserting animals may derive other benefits than remating (reviewed by Clutton-Brock, 1991; Székely et al., 1996). First, desertion may occur when the incubating parent is in a poor condition and thus terminating care may increase the parent's chance of surviving. For example, incubating penguins and seabirds often abort nesting when their body condition is poor (Chaurand and Weimerskirch, 1994; Olsson, 1997; Yorio and Boersma, 1994). Second, parents may also terminate care late in the season when they have to prepare for the nonbreeding period by moulting, for example (Urano, 1992).
Theoretical analyses are invaluable for separating the effects of the various benefits of desertion. In a single-sex model of avian parental care, Webb et al. (submitted) investigated the expected behavior of a parent in relation to its state (body reserves) and time in the breeding season. This theoretical study found that parents with low body reserves deserted because the continuation of care would risk their survival. This decision occurred at any time during the breeding season. However, parents with high body reserves early in the season also deserted, although these parents were not threatened by starvation. Rather, these parents searched for a new mate and attempted to breed again. Finally, some parents deserted at the end of the breeding season when the value of their current offspring was outweighed by the influence of care on their own chances of the surviving the nonbreeding season. Separation of these different reasons for desertion require experimental manipulation of the state of the parent at different times in the season.
The significance of mating opportunity for the evolution of parental care
Understanding mating opportunities may be important for three reasons. First, the option to breed again may influence the duration and intensity of parental care. For example, male cichlid fishes are more likely to terminate care when their mating opportunity is high (Balshine-Earn, 1995; Balshine-Earn and Earn, 1998; Keenleyside, 1983). Similarly, male zebra finches Taeniopygia guttata invest less in their offspring if they have better than average chances of mating and reproducing again (Burley, 1988), and parental care by male red-winged blackbirds Agelaius phoeniceus was reduced when the male had access to a fertile female (Whittingham, 1994). Second, mating opportunities may influence the observed mating patterns. For example, if a male has no chance of attracting another female (because the breeding season is short, the OSR is male biased, or the male is of poor quality), then he may do better by guarding his mate and attending his first female's brood than wasting time and energy on courting females in vain. This has been experimentally demonstrated in insects and in fish (Balshine-Earn, 1995; Vepsalainen and Savolainen, 1995). Third, mating opportunity, in particular the OSR, has been proposed to have a major influence on the intensity of sexual selection and competition for mates (Andersson, 1994; Emlen and Oring, 1977; Kvarnemo and Ahnesjö, 1996). When the OSR deviates from unity, the intensity of mating competition is expected to increase (Colwell and Oring, 1988; Gwynne, 1990; Parker and Simmons, 1996).
Although OSR has often been used as an index of mating opportunity, this relation may not always be correct. First, in many animals it is not straightforward to assess whether an animal is in breeding condition without internal examination. Even in those species in which it is relatively easy to distinguish breeders from nonbreeders, there is a possibility that the animals may not perceive their population members the same way (breeder versus nonbreeder) as the researchers do. Second, the OSR represents only a ratio, and the mating opportunity should depend on the density of males and females in a population as well, because the animals have to find the available individuals. Therefore, a more realistic index of mating opportunity should depend on the absolute number of males and females in the population (Webb et al., submitted). We believe that the experimental assessment of mating opportunities is preferable over simply sampling the animals in a population and deriving their OSR because our measurements such as mating times and reproductive success are based on the responses of the animals.
The observed bias in remating opportunity between males and females in our study may have an implication for the evolution of polyandry (Oring, 1986; Székely, 1996). Classical polyandry (i.e., when a female lays separate clutches for several males in a breeding season) is a rare mating system in birds; the best-known examples are in shorebirds (Clutton-Brock, 1991; Oring, 1986). We propose that the high remating opportunity for deserting females may facilitate the evolution of polyandry. This needs to be investigated by experimental studies of remating opportunities in other shorebird species in which polyandry is facultative. However, the initially malebiased OSR may change over evolutionary time as a result of a response by female shorebirds, such as brood desertion. For example, the proportion of females deserting may increase in the population. Also, females may start to desert their nest and mate at a younger offspring age, such as shortly after egg laying or during incubation (Reynolds and Székely, 1997). In addition, females may compete to return to the breeding ground at an earlier date to secure a mate or a breeding territory (Colwell and Oring, 1988; Reynolds et al., 1986). Taken together, the results of these processes may be that the observed OSR in contemporary populations of polyandrous shorebirds may be equal or female-biased, although the initial condition for the evolution of female desertion and polyandry might have been a male-biased OSR.
In conclusion, we have demonstrated experimentally that female Kentish plovers have better remating opportunities than males. This result is consistent with previous experiments on the Kentish plover. It is also consistent with the observed frequencies of desertion; that is, females desert their brood more often than males in all populations that have been studied so far. We propose that the male-biased OSR is due to a male-biased adult sex ratio and encourage researchers to gather data (which are comparable to our study) in other species. The significance of mating opportunity is that it links mating patterns to parental care. Although this link has been implicitly assumed, it is not fully understood either theoretically or empirically. In particular, we propose that a male-biased OSR facilitated the evolution of polyandry, although, due to a response by female birds to the favorable mating opportunity, the OSR may well be significantly shifted through evolutionary time. Thus the links among observed patterns of parental care, mating system, and OSR may not be as simple as the impression pioneering papers (e.g., Emlen and Oring, 1977) have created.
The project was funded by a Leverhulme Trust [Agrant to A. I. Houston, I.C.C., and J. M. McNamara (F/182/AP) and by an Országos Tudományos Kutatási Alap grant to T.S. (T020036). T.S. was also supported by a Natural Environment Research Council grant to A. I. Houston, I.C.C., and J. M. McNamara (GR3/10957). Rings were provided by P. R. Evans (Durham University) and Radolfzell Vögelwarte, Germany. Ö. Karabaçak (Milli Parklar, Adana), M. Yarar (DHKD, Istanbul), G. Sarigül (DHKD, Tasucu), and V. van den Berk (National Reference Centre for Nature, Wageningen) helped us by providing practical information over the duration of the field study. We thank A. I. Houston and two referees for their comments on the manuscript.
REFERENCES
Balshine-Earn S,
Balshine-Earn S, Earn DJD,
Balshine-Earn S, Earn DJD,
Carlisle TR,
Chaurand T, Weimerskirch H,
Clutton-Brock TH,
Colwell MA, Oring LW,
Emlen ST, Oring LW,
Fraga RM, Amat JA,
Gratto-Trevor CL,
Gwynne DT,
Helms CW, Drury WD Jr,
Keenleyside MHA,
Kvarnemo C, Ahnesjö I,
Lessells CM,
Olsson O,
Parker GA, Simmons LW,
Paton PWC,
Reynolds JD, Colwell MA, Cooke F,
Reynolds JD, Székely T,
Soikkeli M,
Székely T,
Székely T, Lessells CM,
Székely T, Webb JN, Houston AI, McNamara JM,
Székely T, Williams TD,
Urano E,
Uzun G, Yücel M, Yilmaz KT, Berberoglu S,
Vepsalainen K, Savolainen R,
Warriner JS, Warriner JC, Page GW, Stenzel LE,
Whittingham LA,