-
PDF
- Split View
-
Views
-
Cite
Cite
Gordon R. Plague, J Vaun McArthur, Phenotypic plasticity of larval retreat design in a net-spinning caddisfly, Behavioral Ecology, Volume 14, Issue 2, March 2003, Pages 221–226, https://doi.org/10.1093/beheco/14.2.221
Close - Share Icon Share
Abstract
Larval Macrostemum carolina caddisflies construct silken catchnets within protective retreats, often on submerged trees and branches (i.e., snags). In the Savannah River, M. carolina larvae construct three distinct retreats that differ in the configuration of the water entrance hole: (1) at the end of a silken tube, (2) flush with the top of the retreat, and (3) backed by a ∼180-degree silken backstop. To identify the proximate mechanism mediating this retreat polymorphism, we removed larvae of known phenotype from their original retreats and brought them into the laboratory, allowing them to construct new retreats. We found that (1) larvae can construct more than one type of retreat, so variation in this behavior is not under strict genetic control; (2) larvae do not preferentially reconstruct their original retreat design, so these alternative behaviors apparently exhibit little heritability; and (3) larvae primarily construct each phenotype in a particular microhabitat (i.e., “tube” and “backstop” retreats are principally constructed on the downstream half of the snag, and “flush” retreats on the upstream–bottom quadrant). Therefore, the retreat polymorphism in M. carolina is phenotypically plastic and is controlled by microhabitat location or a correlated environmental variable. Most net-spinning caddisflies construct their nets in fairly specific microhabitats. However, behavioral plasticity allows M. carolina larvae to construct retreats all around a snag, thereby reducing potentially intense competition for space with other net-spinning caddisflies. Consequently, this may be the ultimate reason this polymorphism evolved.
Phenotypic polymorphisms have long been of interest to evolutionary biologists (e.g., Darwin, 1859 ; Müller, 1869 ; Wallace, 1864 ), primarily because they are the raw material on which natural selection operates. Discrete polymorphisms are common in many natural populations ( Mayr, 1963 ) and, as such, take a variety of forms, from alternative physiologies (see Bradford and Roff, 1995 ; Semlitsch et al., 1990 ) to morphologies (see Emlen, 1994 ; Hori, 1993 ) to behaviors (see Cade, 1981 ; Lank et al., 1995 ). Whatever the form, distinct polymorphisms are maintained over evolutionary time by one of three proximate mechanisms: (1) alternative alleles at the gene (or genes) controlling the character (see Hori, 1993 ; Lank et al., 1995 ), (2) phenotypic plasticity in response to environmental variability (see Emlen, 1994 ; Semlitsch et al., 1990 ), or (3) a combination of genetic and plastic control, that is, heritable differences that can be environmentally modified (see Bradford and Roff, 1995 ; Cade, 1981 ). Identifying the proximate mechanisms mediating alternative phenotypes not only elucidates the forces that maintain polymorphisms in natural populations but may also help identify the ultimate mechanisms that generate polymorphisms ( Emlen, 1994 ).
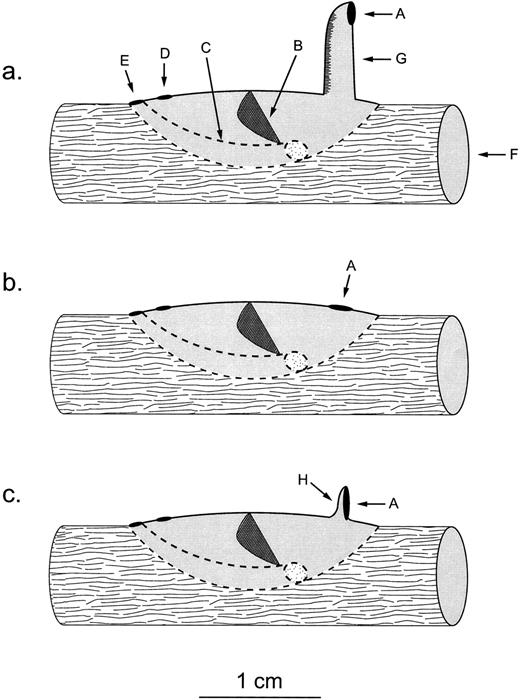
Net-spinning caddisfly larvae construct silken catchnets to filter organic matter from streams. Macrostemum carolina (Trichoptera: Hydropsychidae) net-spinners, which occur throughout the southeastern United States ( Ross, 1944 ), make their catchnets within protective retreats ( Wallace and Sherberger, 1974 ). In coastal-plain streams with shifting sand stream beds, M. carolina larvae primarily construct their retreats on snag habitats (i.e., fallen and submerged trees or branches), gouging the base of their retreats out of the wood and covering the top of the structure with silk. In the Savannah river (GA and SC), these larvae make three different types of retreats, each with a distinct water entrance hole: (1) at the end of a silken tube ( Figure 1a ), (2) flush with the top of the retreat ( Figure 1b ), and (3) backed by an approximately 180° silken backstop ( Figure 1c ; Plague and McArthur, 2000 ). Plague et al. (2001) confirmed that these alternative retreat construction behaviors constitute a polymorphism within a single population; that is, they are not fixed behaviors within reproductively isolated populations. Therefore, we wanted to identify the proximate mechanism mediating this retreat polymorphism. Unfortunately, because snags are generally flexible and often more than 50 cm under relatively turbid water, assessing the in situ location and orientation of a retreat is essentially impossible. Therefore, we removed M. carolina larvae of known phenotype from their original retreats and brought them into the laboratory, allowing them to construct a second retreat. In so doing, our specific objective was to test the following three hypotheses:
(1) There is no correlation between an individual's original retreat phenotype (R1) and second retreat phenotype (R2). If there is an exact correlation (i.e., individuals always reconstruct their R1 design), then M. carolina larvae may be genetically constrained to build a specific retreat, and as such, retreat design is under allelic control (though exact correlation could also derive from nongenetic differences between individuals; see Austad, 1984 ). If there is a non-exact correlation between R1 and R2 retreats (i.e., individuals tend to reconstruct their R1 design, but not always), then retreat design either is an environmentally modifiable heritable behavior or is influenced by prior experience (i.e., larvae reconstruct their R1 design because it is what they “remember” doing). Finally, if there is no correlation between R1 and R2 retreats, then retreat design is apparently phenotypically plastic, and larvae choose their design based solely on the environmental conditions where they settle.
(2) Each R2 phenotype is constructed randomly around a snag. If retreat phenotypes are not randomly constructed around a snag, then each may be adapted for a particular microgeographic location. If so, and if retreat choice is not under strict allelic control (see above), then this may be the environmental condition mediating retreat choice. Based on the unique architecture of each retreat design, Plague and McArthur (2000) suggested that “tube” retreats may primarily occur on downstream snag locations, reaching over the top or bottom of the snag to face into the current, “backstop” retreats may primarily occur on the tops and bottoms of snags, with the silken backstop diverting water into the retreat, and “flush” retreats may primarily occur on upstream snag locations, facing directly into the water current.
(3) Each R2 phenotype is constructed randomly with respect to water flow velocity. If each retreat phenotype is preferentially constructed in a specific water velocity microhabitat, then each may be adapted for that velocity. Plague and McArthur (2000) proposed that backstop and tube retreats may function as Pitot tubes (instruments that physically pull water owing to the pressure differential created by the orientation of their entrance and exit holes [vertical and horizontal, respectively, to the water flow]), drawing more water and food through their retreats than would flow through passively. As such, these retreats may be adapted for relatively low-flow microhabitats and flush retreats for high-flow microhabitats. If so, then flow velocity may be the environmental condition mediating retreat choice if the polymorphism is not under strict allelic control (see above).
METHODS
Artificial stream channels
We constructed three recirculating artificial stream channels, modeled after a design in Vogel and LaBarbera (1978 , see their Figure 1). These channels consisted of a Plexiglas trough (76 × 13 × 19 cm, L × W × H) and a PVC return pipe (7.6-cm diam). Each chamber held approximately 20 l of water. Water was recirculated by using a 6.4-cm diam impeller attached to a 1/15-horsepower motor (Fasco Industries, Model D234). The trough contained two upstream collimators to linearize the water flow (each an array of approximately 3-cm-long plastic drinking straws), two 8-cm-long snag pieces used as substrate for the larvae (arranged perpendicular to the water flow and one above the other in the trough), and a downstream screen to catch dislodged insects. The stream channels were housed in an environmental chamber in which the day length and temperature were maintained at ambient Savannah river conditions. A shade cloth was placed over each trough to reduce the light level and make them more similar to the somewhat turbid Savannah river. Because M. carolina larvae filter organic matter from the water column, we used water from the Savannah River in the stream channels, refreshing it at least every 3 days.
Larval rearing
We attempted to collect 30 M. carolina larvae of each retreat phenotype for each of five rearing bouts ( Table 1 ). All collections were made from snags in the Savannah River, approximately 205 km upstream of the river's confluence with the Atlantic ocean (the access point was Johnson's Landing, Allendale County, SC). On all dates, caddisflies were transported back to the laboratory on ice and held overnight at 7°C. All individuals of a single retreat morph were reared in one of the three artificial stream channels. We initially placed half of the individuals of each morph on each snag piece in the designated stream channel, and subsequently replaced dislodged individuals alternately on each snag. The channels were usually monitored every day to replace dislodged individuals and to measure the water flow velocity approximately 0.5-cm upstream of all snags (using a Nixon StreamFlo flow meter). We averaged these daily velocity measurements over the entire rearing bout to get a mean velocity for each snag. Each rearing bout lasted 2–3 weeks.
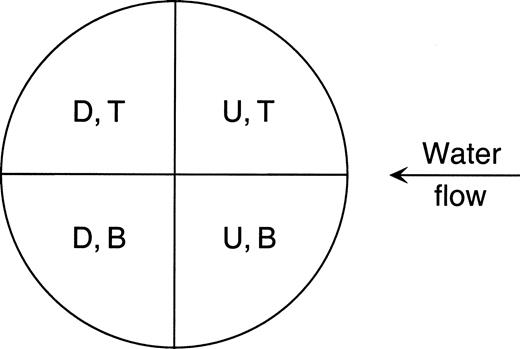
On completion of each rearing bout, we recorded the phenotype and location of each retreat, and whether a net was constructed. The location of each retreat was assigned to one of four snag quadrants: upstream–top, upstream–bottom, downstream–bottom, and downstream–top ( Figure 2 ).
Data analyses
We tested the three hypotheses (see above) by using contingency table χ 2 tests, performed with the PROC FREQ procedure in SAS ( SAS Institute, 1996 ). In testing hypothesis 1, we performed a three × three cross-tabulation of R1 versus R2, testing for randomness between them. In testing hypothesis 2, we grouped snag quadrants and compared the number of each R2 morph on the upstream half of the snag to the number on the downstream half, and the number on the top half of the snag to the number on the bottom half. We tested these observations against the null hypothesis of an equal distribution of larvae on both halves of the snag (i.e., upstream and downstream, top and bottom). In testing hypothesis 3, we divided the 30 snags (three channels × two snags per channel × five collection dates) into three equal groups based on the mean water velocity each was exposed to (i.e., the 10 lowest, middle, and highest velocities), and compared the number of each R2 morph in each flow velocity category with the null hypothesis of an equal distribution of larvae among categories.
We assessed retreat construction trends over time by using Cochran-Armitage trend tests, performed with the Proc Freq procedure in SAS ( SAS Institute, 1996 ). To evaluate these trends, we combined the backstop and tube individuals to meet the χ 2 test assumptions of the Cochran-Armitage trend test ( Cochran, 1954 ). We also measured the amount of R2 retreat variation attributable to larval age and microhabitat location by performing logistic regression analyses integrating both, using the PROC REG procedure in SAS. In these analyses, the dependent variable was the ln of the probability of building each R2 retreat on each half of the snag (i.e., upstream versus downstream, top versus bottom) as a function of date.
RESULTS
Not all M. carolina larvae used in this experiment constructed second retreats. Those that did not either died during the rearing bout ( N = 103), drifted off the snag and were on the screen at the end of the bout ( N = 19), or just covered themselves with silk without constructing a formal retreat ( N = 209). Because it was sometimes difficult to objectively discern whether an individual simply covered itself with silk or constructed a flush retreat but had not yet spun a net, we only counted as retreats those structures that contained nets ( N = 101).
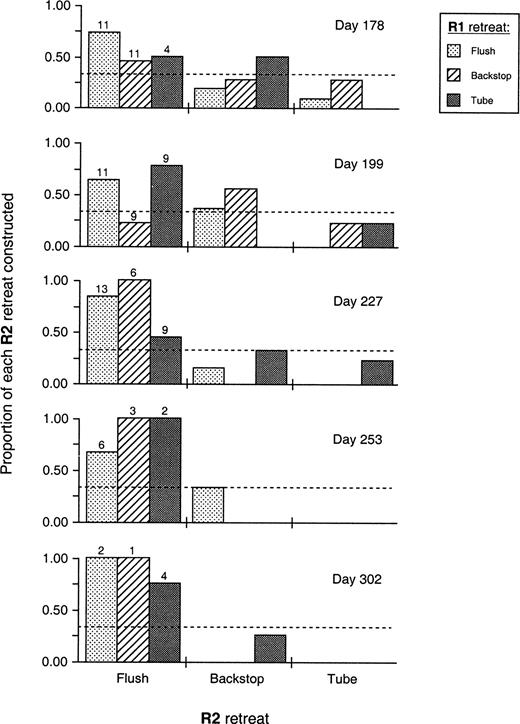
Individuals of each R1 phenotype were capable of reconstructing all retreat designs ( Figure 3 ). However, larvae progressively constructed significantly fewer retreats over time (df = 4, p <.001), and these retreats were progressively more likely to be the flush phenotype (df = 4, p =.031) ( Figure 3 ). At any rate, larvae apparently did not base their R2 design on their R1 phenotype (df = 4, G = 0.179), so we cannot reject hypothesis 1 (i.e., larvae evidently choose their R2 phenotype at random with respect to R1).
Larvae did not randomly construct their retreats on the upstream and downstream halves of the snag (df = 2, G < 0.001), or on the top and bottom halves of the snag (df = 2, G = 0.038). Flush retreats were preferentially built on the upstream half, whereas backstop and tube retreats were preferentially built on the downstream half ( Table 2 ). Flush retreats were also preferentially built on the bottom half of the snag, whereas backstop and tube retreats did not exhibit a strong top or bottom tendency ( Table 2 ). Therefore, we must reject hypothesis 2 (i.e., larvae do not construct each retreat phenotype randomly around a snag).
Flush and backstop R2 retreats exhibited several significant microhabitat location trends over time (i.e., p ≤.05), although the tube retreats did not exhibit any such trends. Specifically, flush retreats were constructed progressively less often on the top half of the snag ( r = −.873, F = 41.48, df = 1,13, p <.001), whereas backstop retreats were constructed progressively less often on the top ( r = −.515, F = 4.70, df = 1,13, p =.049), bottom ( r = −.739, F = 15.63, df = 1,13, p =.002), and downstream ( r = −.683, F = 11.39, df = 1,13, p =.005) halves of the snag. Also, larvae that did not construct retreats (but simply covered themselves with silk) progressively settled significantly more often on the downstream half of the snag ( r =.552, F = 5.70, df = 1,13, p =.033).
Although the flush and tube retreats tended to be constructed on snags exposed to medium water flow velocities (low, 12.49–23.07 cm/s; medium, 23.12–25.70 cm/s; and high, 26.57–40.88 cm/s) ( Table 3 ), retreat phenotype apparently was not correlated to flow velocity (df = 4, G = 0.125). Therefore, we cannot reject hypothesis 3 (i.e., larvae construct each retreat phenotype randomly with respect to water flow velocity).
Discussion
There was no correlation between the retreat designs that M. carolina larvae constructed in the Savannah river and those they reconstructed in the laboratory. Consequently, this retreat polymorphism is not genetically hardwired and is therefore not mediated by alternative alleles at a Mendelian gene. Also, although we did not use parent-offspring regressions (see Roff, 1986 ) or full-sib (see Brodie and Brodie, 1990 ) or half-sib comparisons (see Collins et al., 1999 ) to directly measure the heritability of retreat design, we did indirectly measure heritability by assessing this behavior's repeatability ( Ehrman and Parsons, 1976 ). Because of the noncorrelation (and nonrepeatability) between R1 and R2 retreats, we must conclude that these alternative behaviors are not due to heritable differences between individuals. By default, therefore, the alternative retreats in M. carolina are apparently phenotypically plastic; that is, all larvae are able to construct all three retreats, and they “choose” their retreat design based on the environmental conditions where they settle. This conclusion is supported by our finding that each phenotype is preferentially constructed in a particular location around a snag, which is apparently the environmental cue controlling this plasticity (or is correlated to the conditional control mechanism, see below). Tube and backstop retreats are primarily built on the downstream half of the snag, whereas flush retreats are primarily built on the upstream–bottom quadrant. These observations are consistent with the microhabitat location predictions made by Plague and McArthur (2000) for all but the backstop retreats (i.e., that flush retreats primarily occur on upstream snag locations, tube retreats on the downstream side of snags, and backstop retreats on the tops and bottoms of snags), although we were unable to adequately assess “top” and “bottom” locations because of our delineation of snag quadrants (see Figure 2 ). Therefore, the function of the silken tubes and backstops may be simply to divert water (and therefore food) through the retreat, thereby opening up snag microhabitats that are not exposed to direct water flow and that otherwise would be uninhabitable for the flush design. In addition, the identity in location preference for tube and backstop morphs suggests that these may represent a phenotypic continuum of a single behavior; that is, backstop retreats may also be designed to reach over the top of snags, although they may primarily be in locations that do not require a long reach.
If larvae are strictly guided by microhabitat location when choosing their retreat design, then all retreats of each design should cluster in the same general vicinity on a snag. Because this was not the case, retreat choice in M. carolina may be mediated by an alternate environmental control mechanism that is related to microhabitat location. This mechanism may be water flow velocity. Although the M. carolina retreat phenotypes were not correlated to flow velocity at our relatively coarse level of measurement, numerous flow microhabitats undoubtedly exist all around a snag ( Hart et al., 1996 ). Nonetheless, flow microhabitats are probably largely correlated to microhabitat location, with upstream snag locations generally experiencing greater velocities than downstream locations. This likely correlation, coupled with the tendency for retreat phenotypes to cluster in particular locations but without exact fidelity, suggests that M. carolina larvae may use microflow, at least partially, as their retreat choice guide. If so, then the clustering of tube and backstop retreats on downstream locations is consistent with the hypothesis that these retreats function as Pitot tubes (see above) and therefore are adapted to relatively low-flow conditions ( Plague and McArthur, 2000 ).
In general, M. carolina larvae progressively reconstructed fewer retreats over time, and those that were constructed progressively tended to be the flush phenotype. As the time for pupation nears, larvae may be less and less willing to expend energy to construct new retreats, “deciding” that the construction costs outweigh the potential benefits of making a new retreat. Larvae that choose to construct may prefer the flush phenotype because it is presumably the most inexpensive to make, with backstop and tube retreats requiring progressively greater expenditures of silk and time, equating to lost feeding time and increased exposure to predators (unequal construction expense may also explain why flush retreats were consistently the most common design in our rearing experiments; i.e., larvae presumably chose microhabitats suitable for the “inexpensive” flush retreats if they were available). Unfortunately, the trends for flush and backstop retreats to progressively become more rare in particular snag microhabitats (i.e., flush on the top half; backstop on the top, bottom, and downstream halves) are currently inexplicable. However, the trend for nonretreat constructing larvae to progressively favor the downstream side of the snag may be a selectively advantageous strategy. If these larvae are simply trying to expend the least energy possible while waiting to begin pupation (which seems progressively more likely as the time for pupation nears), then the downstream side is presumably the best and safest location because it should provide the lowest likelihood of accidental dislodgement or death from drifting debris.
Polymorphic traits that evolve in stable environments frequently have a strong genetic component, while those evolving in unstable environments are often controlled by phenotypic plasticity ( Smith and Skúlason, 1996 ). Coastal plain streams generally have very unstable water depths, often fluctuating more than 200% in a univoltine insect's larval lifetime (e.g., at a gauging station approximately 14 km downstream of our collecting site, the Savannah river fluctuated between 1.3–3.9 m in 1999; United States Geological Service, 2000 ).
When water levels fall, exposed net-spinning caddisflies must drop to lower snags to find suitable locations for their new nets. However, finding a suitable and unoccupied new location could be very difficult because net-spinners generally construct their nets in fairly specific microhabitats ( Wallace and Merritt, 1980 ), and because their densities are often extremely high on snags in coastal plain streams (e.g., more than 100,000 individuals/m 2 of snag habitat in the Savannah River; Cudney, 1979 in Wallace, 1982 ). Consequently, if all suitable habitats are occupied, a net-spinner's options are to (1) attempt aggressive removal of a resident caddisfly from its location ( Glass and Bovbjerg, 1969 ; Hildrew and Townsend, 1980 ; Jansson and Vuoristo, 1979 ), (2) drift downstream in the hopes of finding a better snag ( Wallace, 1975 ), or (3) settle in a suboptimal habitat ( Georgian and Thorp, 1992 ). Unfortunately, these are all risky options to the caddisfly's growth or survival. M. carolina larvae's ability to construct multiple retreats adapted to multiple microhabitats is undoubtedly selectively advantageous because relatively many snag locations are available to them, thereby decreasing the necessity for the three risky behaviors above. Subsequently, reducing competition for space may be the ultimate cause of the phenotypic plasticity in their retreat design.
As we have argued, backstop and tube retreats must require more silk and time to construct than flush retreats, although we have no data on the time and energy required to construct each one. If there is no added fitness benefit to making the more expensive retreats, then this differential construction cost supports the proposal that competition for space is the ultimate cause of the retreat polymorphism of M. carolina . In general, alternative plastic traits usually result from one of two selective regimes, environmental heterogeneity or intraspecific competition, although competition is the likely cause when the phenotypic alternatives are unequally profitable (although competitors may capitalize on environmental heterogeneity to reduce competition; West-Eberhard, 1989 ). Therefore, the retreat polymorphism in M. carolina likely resulted from larvae attempting to escape competition for space, although we suspect that interspecific competition with other net-spinning caddisflies may have been as important as intraspecific competition.
The larval retreat morphs of Macrostemum carolina : (a) “tube retreat,” (b) “flush retreat,” and (c) “backstop retreat.” A indicates water entrance hole; B, net; C, larval chamber; D, net chamber water exit hole; E, larval chamber water exit hole; F, snag; G, silken tube; and H, silken backstop. (This is amended from Figure 1 in Plague and McArthur, 2000 .)
Side-view (left end) of an experimental snag piece, showing how the snag was divided into quadrants. U indicates upstream; D, downstream; T, top; and B, bottom
Proportion of each R2 retreat type constructed by each R1 phenotype for each rearing bout. Day indicates the day of that generation's life on the collection date (assuming the generation started on 1 May, from Cudney and Wallace, 1980 ). The number above each Flush R2 bar is the number of larvae constructing retreats for each respective R1 at each date. The dashed line on each rearing bout graph is the expected frequency of each R2 retreat, if larvae choose their R2 retreat at random with respect to their R1
Collection information for each rearing bout.
| . | . | . | Number collected . | ||
|---|---|---|---|---|---|
| Rearing bout . | Collection date . | Corresponding day of life . | Flush . | Backstop . | Tube . |
| 1 | 10/27/99 | 178 | 30 | 30 | 30 |
| 2 | 11/17/99 | 199 | 30 | 30 | 30 |
| 3 | 12/15/99 | 227 | 30 | 30 | 30 |
| 4 | 1/10/00 | 253 | 31 | 23 | 17 |
| 5 | 2/28/00 | 302 | 30 | 31 | 30 |
| . | . | . | Number collected . | ||
|---|---|---|---|---|---|
| Rearing bout . | Collection date . | Corresponding day of life . | Flush . | Backstop . | Tube . |
| 1 | 10/27/99 | 178 | 30 | 30 | 30 |
| 2 | 11/17/99 | 199 | 30 | 30 | 30 |
| 3 | 12/15/99 | 227 | 30 | 30 | 30 |
| 4 | 1/10/00 | 253 | 31 | 23 | 17 |
| 5 | 2/28/00 | 302 | 30 | 31 | 30 |
Collection information includes the date of collection, the corresponding day of that generation's life (assuming the generation started on 1 May; from Cudney and Wallace, 1980 ), and the number of individuals of each retreat phenotype collected.
Collection information for each rearing bout.
| . | . | . | Number collected . | ||
|---|---|---|---|---|---|
| Rearing bout . | Collection date . | Corresponding day of life . | Flush . | Backstop . | Tube . |
| 1 | 10/27/99 | 178 | 30 | 30 | 30 |
| 2 | 11/17/99 | 199 | 30 | 30 | 30 |
| 3 | 12/15/99 | 227 | 30 | 30 | 30 |
| 4 | 1/10/00 | 253 | 31 | 23 | 17 |
| 5 | 2/28/00 | 302 | 30 | 31 | 30 |
| . | . | . | Number collected . | ||
|---|---|---|---|---|---|
| Rearing bout . | Collection date . | Corresponding day of life . | Flush . | Backstop . | Tube . |
| 1 | 10/27/99 | 178 | 30 | 30 | 30 |
| 2 | 11/17/99 | 199 | 30 | 30 | 30 |
| 3 | 12/15/99 | 227 | 30 | 30 | 30 |
| 4 | 1/10/00 | 253 | 31 | 23 | 17 |
| 5 | 2/28/00 | 302 | 30 | 31 | 30 |
Collection information includes the date of collection, the corresponding day of that generation's life (assuming the generation started on 1 May; from Cudney and Wallace, 1980 ), and the number of individuals of each retreat phenotype collected.
The number of each retreat phenotype constructed on the upstream versus downstream halves of the snag, and the top versus bottom halves of the snag.
| Retreat . | Upstream . | Downstream . | Top . | Bottom . |
|---|---|---|---|---|
| Flush | 42 | 23 | 20 | 45 |
| Backstop | 5 | 14 | 11 | 8 |
| Tube | 0 | 6 | 4 | 2 |
| Retreat . | Upstream . | Downstream . | Top . | Bottom . |
|---|---|---|---|---|
| Flush | 42 | 23 | 20 | 45 |
| Backstop | 5 | 14 | 11 | 8 |
| Tube | 0 | 6 | 4 | 2 |
Retreats built on the ends of the snags were excluded from this analysis.
The number of each retreat phenotype constructed on the upstream versus downstream halves of the snag, and the top versus bottom halves of the snag.
| Retreat . | Upstream . | Downstream . | Top . | Bottom . |
|---|---|---|---|---|
| Flush | 42 | 23 | 20 | 45 |
| Backstop | 5 | 14 | 11 | 8 |
| Tube | 0 | 6 | 4 | 2 |
| Retreat . | Upstream . | Downstream . | Top . | Bottom . |
|---|---|---|---|---|
| Flush | 42 | 23 | 20 | 45 |
| Backstop | 5 | 14 | 11 | 8 |
| Tube | 0 | 6 | 4 | 2 |
Retreats built on the ends of the snags were excluded from this analysis.
The number of each retreat phenotype constructed in low, medium, and high water flow velocities a .
| Retreat . | Low . | Medium . | High . |
|---|---|---|---|
| Flush | 17 | 34 | 16 |
| Backstop | 8 | 11 | 5 |
| Tube | 0 | 8 | 2 |
| Retreat . | Low . | Medium . | High . |
|---|---|---|---|
| Flush | 17 | 34 | 16 |
| Backstop | 8 | 11 | 5 |
| Tube | 0 | 8 | 2 |
a Low water flow velocity: 12.49–23.07 cm/s; medium: 23.12–25.70 cm/s; high: 26.57–40.88 cm/s.
The number of each retreat phenotype constructed in low, medium, and high water flow velocities a .
| Retreat . | Low . | Medium . | High . |
|---|---|---|---|
| Flush | 17 | 34 | 16 |
| Backstop | 8 | 11 | 5 |
| Tube | 0 | 8 | 2 |
| Retreat . | Low . | Medium . | High . |
|---|---|---|---|
| Flush | 17 | 34 | 16 |
| Backstop | 8 | 11 | 5 |
| Tube | 0 | 8 | 2 |
a Low water flow velocity: 12.49–23.07 cm/s; medium: 23.12–25.70 cm/s; high: 26.57–40.88 cm/s.
We thank A. Lindell and G. Novak for their help in the field, T. Philippi and C. Tuckfield for statistical advice, and T. Glenn and two anonymous reviewers for insightful comments on the manuscript. The CMC group, especially D. Maffett, provided invaluable assistance constructing the artificial stream channels. This research was supported by Financial Assistance Award Number DE-FC09-96SR18546 from the U.S. Department of Energy to the University of Georgia Research Foundation.
REFERENCES
Austad SN,
Bradford MJ, Roff DA,
Brodie ED, III, Brodie ED, Jr,
Cade WH,
Collins RD, Jang Y, Reinhold K, Greenfield MD,
Cudney MD,
Cudney MD, Wallace JB,
Emlen DJ,
Georgian T, Thorp JH,
Glass LW, Bovbjerg RV,
Hart DD, Clark BD, Jasentuliyana A,
Hildrew AG, Townsend CR,
Hori M,
Jansson A, Vuoristo T,
Lank DB, Smith CM, Hanotte O, Burke T, Cooke F,
Plague GR, McArthur JV,
Plague GR, Mulvey M, Glenn TC, McArthur JV,
Roff DA,
SAS Institute, Inc.,,
Semlitsch RD, Harris RN, Wilbur HM,
Smith TB, Skúlason S,
United States Geological Service,,
Wallace AR,
Wallace JB,
Wallace JB,
Wallace JB, Merritt RW,
Wallace JB, Sherberger FF,