-
PDF
- Split View
-
Views
-
Cite
Cite
Klaudia Witte, Hamilton E. Farris, Michael J. Ryan, Walter Wilczynski, How cricket frog females deal with a noisy world: habitat-related differences in auditory tuning, Behavioral Ecology, Volume 16, Issue 3, May/June 2005, Pages 571–579, https://doi.org/10.1093/beheco/ari032
Close - Share Icon Share
Abstract
Cricket frogs (Acris crepitans) occupy a variety of acoustically different habitats ranging from pine forest to open grassland. There is geographic variation in their calls and the tuning of their basilar papilla (BP) correlated with habitat. Here, we characterize the spectral content of environmental noise from two habitats, one a pine forest (Stengl) and one a grassland (Gill) habitat. We then used rounded exponential filter functions based on the mean tuning of auditory fibers in Stengl and Gill females to model the BP tuning characteristics of an average female from the two cricket frog populations occupying those habitats to compare their ability to filter out environmental noise. Noise recordings were made at both sites from 1800 to 2400 h on multiple nights throughout a breeding season (March through early August). Noise spectra were similar at both sites. Cross-correlation analyses of the sampled noise indicated that noise spectra were consistent throughout the night and varied little over the season other than during the month of May. The model auditory filter simulating an average Stengl female was significantly better than one simulating an average Gill female at filtering environmental noise at both sites. Previous work had shown that cricket frog calls suffered greater attenuation and degradation in the Stengl site than the Gill site but that the male calls from Stengl frogs suffered less attenuation and less degradation than Gill calls during transmission through both habitats. These new results demonstrate that frogs from the more acoustically challenging Stengl habitat have enhanced both the sender and receiver portions of their communication system, evolving calls that transmit better and auditory filters that better eliminate noise.
Animal communication systems often involve senders and receivers that interact over distances well in excess of their body size. In these systems, the signal must propagate through the environment, and the characteristics of that environment can seriously limit the effectiveness of the signal by attenuating it, degrading it, or masking it with extraneous signals (noise) (Brenowitz, 1986; Marten and Marler, 1977; Morton, 1975; Richards and Wiley, 1980; Ryan and Kime, 2003; Wiley and Richards, 1978, 1982). For the communication to be effective, a receiver must be able to recognize a signal and discriminate its characteristics in the face of these environmental challenges to the signal's integrity. As many such communication systems mediate mate attraction, the environmental characteristics can represent strong selective pressures on the form of the communication signal and on the characteristics of the behavior or physiology of the sender and the receiver. To maximize the efficacy of communication, the sender's signal may evolve to improve signal detectability and fidelity, the receiver's sensory physiology may evolve to maximize signal detection and minimize masking by extraneous noise, or both sender and receiver can evolve with the sender matching the signal to the environment and the receiver evolving a sensory system that compensates for environmental problems.
Many studies have investigated the influence of environmental characteristics on the sender by examining the form of acoustic (e.g., Gish and Morton, 1981; Ryan and Sullivan, 1989; Ryan et al., 1991; Waser PM and Waser MS, 1977) and visual (Boughman, 2001; Endler, 1991, 1992, 1995; Fleishmann, 1992; Marchetti, 1993; Reimchen, 1989) communication signals used in different habitats. These studies have indicated that for both visual and acoustic communication, signals evolve in response to environmental selection pressures. In addition, variation in the tuning of the receiver's auditory or visual system that matches the signal's variation has been identified in many cases at both the species and the intraspecific population level (reviewed in Gerhardt and Schwartz, 2001; Wilczynski and Ryan, 1999), and this may be in part a strategy to enhance signal reception, although such a match in the peripheral sensory system does not completely account for discrimination among, or preferences for, signals. Despite a rich body of work on signal variation correlated specifically to environmental factors, that is, on variation in the behavior of the sender, relatively little attention has been paid to potential variation in the receiver in relation to habitat characteristics. Some notable exceptions are work on visual pigments in a variety of fish species, which has identified variation in retinal pigment absorption correlated with optical properties of the habitat (Cummings and Partridge, 2001; Lythgoe et al., 1994). Similarly, Lall et al. (1980) found that firefly species differed in both bioluminescence peaks and visual receptor spectral sensitivities depending on the time of night during which they are active and that these differences enhanced the signal-to-noise ratio in their particular habitats. In the auditory domain, Langemann et al. (1998) reported that the thresholds and critical masking ratios of the high-frequency portion of the great tit's hearing range made it particularly well suited to detect its relatively high-frequency call in the type of wind-generated noise characterizing deciduous forest. In no case, however, has there been an investigation of geographic variation in auditory receiver characteristics that might enhance call reception or discrimination in different environments. In this study, we first characterized the spectral composition of environmental noise in different cricket frog habitats and then examined whether the noise-filtering characteristics of the peripheral auditory system vary between populations residing in those different habitats.
Our study used the cricket frog, Acris crepitans, as the subject of the investigation. Cricket frogs are small anuran amphibians that occupy a broad geographic range across the southern and eastern US. Males produce an advertisement call composed of a series of short, pulsatile, click-like components organized in a call group (Ryan and Wilczynski, 1991). Females use the call for mate recognition (Nevo and Capranica, 1985; Ryan and Wilczynski, 1988; Ryan et al., 1992; Witte et al., 2001), and it is also the basis for a variety of male-male agonistic interactions (Burmeister et al., 1999a,b, 2002; Perrill and Shepherd, 1988; Wagner, 1990). A. crepitans populations occupy a range of habitat types that vary from densely wooded pine forests to open grassland. The calls of the species vary geographically, with consistent differences between populations in forest and open habitats (Ryan and Wilczynski, 1991; Wilczynski and Ryan, 1999). Calls from populations in forest habitats are higher in dominant frequency, shorter in duration, and faster in pulse rate than those from the less acoustically cluttered open habitats. The forest habitat is more acoustically challenging in that cricket frog calls degrade and attenuate faster in them (Ryan et al., 1991; Venator, 1999). Previous transmission experiments (Ryan et al., 1991) have indicated that the calls of forest populations transmit with much less degradation in either habitat than those from open grassland populations. Their advantage is much more significant in the forest habitat. The open habitat has much less of a degradative impact on either type of call, but there too the forest habitat call propagates with slightly less degradation. These results suggest that habitat acoustics have provided a selective force that has shaped the signals used in this communication system such that calls from the more acoustically challenging forest habitats are structured to improve transmission fidelity.
Geographic variation is also seen in the tuning of the peripheral auditory system in cricket frogs (Keddy-Hector et al., 1992; Nevo and Capranica, 1985; Wilczynski and Ryan, 1999; Wilczynski et al., 1992). The cricket frog call is characterized by a single peak, or dominant, frequency that ranges from 2.8 to 4.2 kHz among populations and has little or no energy less than 2.0 kHz. This places it within the sensitivity band of the basilar papilla (BP), the higher tuned of the two auditory papillae possessed by anuran amphibians (Wilczynski and Capranica, 1984). (The other auditory end organ, the amphibian papilla has receptors tuned to a range of frequencies from approximately 100–1200 Hz.) The average tuning of the BP in each population is close to the average dominant frequency of the call produced by males in that population (Wilczynski et al., 1992). Just as calls are generally higher in frequency in forest populations, so is the tuning of the BP (Wilczynski et al., 1992). There is also a sex difference in tuning, with males tuned lower than females (Wilczynski et al., 1992).
The match between call-dominant frequency and BP tuning no doubt enhances a frog's ability to detect the signal by making its auditory system most sensitive to the spectral frequencies of the call. A second way that the receiver could in principle enhance signal detection is to structure its filter characteristics to reduce masking by acoustical noise. As in other vertebrates, the anuran auditory system is susceptible to noise masking (Ehret and Capranica, 1980; Gerhardt and Klump, 1988; Narins, 1982, 1987; Wollerman and Wiley, 2002). The range of noise spectra that will mask reception of a signal is determined by the band-pass features of the auditory neurons, which are reflected in the shape of auditory tuning curves. Extraneous acoustic noise with spectral frequencies within the boundaries of the tuning curve will be detected by the auditory system along with the signal of interest (the advertisement call) and thereby mask it, while acoustic noise outside the limits of the tuning curve will not. Therefore, it is obvious that variation in the width of the tuning curve and the placement of its center frequency and boundaries will significantly affect the amount of potential masking noise that will invade the auditory system. Furthermore, if noise characteristics differ among habitats, the filtering properties of tuning curves may be more or less effective at filtering environmental noise.
It is clear from previous studies (Nevo and Capranica, 1985; Ryan and Wilczynski, 1988; Wilczynski and Ryan, 1999; Wilczynski et al., 1992) that the tuning of the peripheral auditory system differs among cricket frog populations. What remains unexamined in this species, or any other, is whether the band-pass characteristics of the peripheral auditory system fibers differ among populations in any way related to habitat acoustics. Specifically, it is unknown whether peripheral auditory-filtering characteristics evolved in different habitats to enhance noise filtering in those habitats. We compared the performance of the average filtering characteristics of female peripheral auditory fibers of two cricket frog populations occupying the two basic habitat types in which this species occurs, pine forest and open grassland, using natural noise recorded in both habitats. Three possibilities exist. First, noise-filtering characteristics of auditory fibers could be locally adapted, which would be evident by each population's auditory system filtering best in its own habitat, and better than the filter from the population in the other habitat. Second, one filter could be absolutely better than any other, which would be evident in it being better at filtering noise in both its habitat and in the other population's habitat. Third, the null hypothesis is that the two filters are not significantly different in their performance in either habitat.
We performed this analysis by modeling the peripheral tuning curves of cricket frog females from a pine forest population and an open grassland population. We constructed an average tuning curve for each population, based on the mean values of several tuning curve characteristics, and modeled each as a rounded exponential (roex) filter (see below). We then used these filters to determine how well would an average female from each of the populations perform in filtering acoustic noise in each of the two habitats. We did this by assessing the performance of both filters in filtering noise signals obtained from both habitats throughout the breeding season.
MATERIALS AND METHODS
Recording the background noise
We recorded the background noise at two different field sites: at a semipermanent pond in an open grassland area (Gill Ranch, Travis County, Texas, USA) and at a permanent pond in a pine forest (Stengl Ranch, Bastrop County, Texas, USA) where cricket frogs normally call. We recorded the background noise from 3 March to 9 August 1996 between 1800 and 2400 h on two consecutive nights every 2 weeks. We recorded the first minute of each hour. We attached a microphone (Radio Shack) 20 cm above the ground to a tripod near the pond at each site. The microphone had a windscreen and extra foam that deadened the noise of raindrops falling on the microphone. Additionally, a plastic hat served as a protection against rain. The microphone was connected to a timer (Vox clock), tape recorder (Aiwa), and battery supply. Identical equipment was used at each site.
The chorus size at both sites was small, and males called sporadically during the season when we recorded our noise samples. The recording microphone was carefully placed to avoid nearby calling males at both sites. Furthermore, noise was taken in short, random 1-s increments during the recording period, which were then averaged, making any significant or systematic contribution of conspecific calls to the noise relatively rare. Inspection of the resultant noise spectra confirmed this; samples that contained significant conspecific calls were not used. Therefore, while we cannot completely exclude a contribution of conspecific calls to the sampled noise, most of the noise energy derived from other sources.
We digitized the recorded noise with SIGNAL Version 3.0 (Engineering Design, Belmont, Massachusetts, USA). We divided each minute into two 30-s intervals and created an average signal lasting 1 s for each 30-s interval of recorded noise by acquiring a randomly chosen 1-s sample of the noise within that half minute 10 times, adding them, and dividing the resultant sum by 10. For each average signal we calculated a Fast Fourier transform (FFT). As the noise was recorded on 24 nights at each site throughout the breeding season (March through August), we therefore obtained a maximum of 336 digitized noise samples from each site. We used for the analysis a sample of 325 average signals from Gill Ranch and 328 noise samples from Stengl Ranch, as we discarded some samples due to anthropogenic noise (e.g., airplane traffic) during recordings.
Analysis of noise variation
We performed cross-correlations of ambient noise within each site to examine variation in noise spectra throughout a night from 1800 to 2400 h and throughout the breeding season. For the analysis of noise variation over the season, we took an average noise signal of each hour in two consecutive nights every 2 weeks from March through early August. We then cross-correlated the average noise signals of each hour and month with the noise signals in March in that habitat (n = 14 average signals from Gill Ranch, n = 13 average signals from Stengl Ranch). A cross-correlation value of 1.0 indicates identical noise composition compared to the March noise. For the analysis of noise variation through a night, we took the average noise signal at 1800, 2100, and 2400 h of all 23 and 24 nights sampled throughout the season from the Gill Ranch and Stengl Ranch respectively. We then cross-correlated the signals from 2100 to 2400 h with the noise at 1800 h. A cross-correlation value of 1.0 indicates identical noise composition compared to the noise at 1800 h.
Constructing filter functions
We constructed roex filters representing the average tuning characteristics of peripheral BP auditory fibers in females from the two study sites. A roex filter is an idealized mathematical function that accurately represents the bandwidth characteristics and high- and low-frequency slopes of an auditory filter as a roex function centered at the filter's characteristic frequency; the rounding and exponential components of the function accommodate the shape of the tuning curve's tip and its nonlinear flanks, respectively. This function, which has proved more accurate than linear or rectangular models, has been used to model auditory filters across disparate taxa including humans, dolphins, and crickets (Farris and Hoy, 2002; Finneran et al., 2002; Patterson et al., 1982). The filter functions were calculated using a method described by Patterson et al. (1982; see also Moore et al., 1990). Each filter was modeled using the tuning curves of VIII nerve auditory fibers presumed to originate from the BP. The tuning curves were based on previously published neurophysiological data from single-unit recordings from the VIII cranial nerve in females from the two populations (Keddy-Hector et al., 1992; Wilczynski et al., 1992). Not all the data from the previous studies were used, only data from individuals from which clear, single-unit recordings were obtained from multiple BP fibers for which all the requisite tuning parameters could be ascertained. For females from the Gill Ranch site (open grassland), we used the mean best excitatory frequency (BEF), Q10, and Q20 (bandwidth divided by the BEF at 10 and 20 dB above threshold respectively) of VIII nerve fibers from three females. For the Stengl Ranch site (pine forest), we used the same parameters from 10 females. In each case, the values from multiple BP fibers within an individual were averaged to yield mean values for that female. Female means were then averaged across females within a population to yield a population mean for each site. As VIII nerve fiber tuning curves are not symmetrical around the BEF (the high-frequency flanks are steeper than the low-frequency flanks), the boundaries of the tuning curve were adjusted accordingly using the average asymmetry in the recorded fibers in the two populations. The tuning curve parameters were then used to construct roex filters for each population using Mathcad (Mathsoft, Cambridge, Massachusetts, USA).
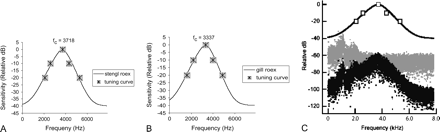
Graphic representation of calculated roex filters from the Stengl (A) and Gill (B) neurophysiological data. Lines indicate the boundaries of the roex filter, symbols along the lines indicate average tuning curve points from the neural data; fc = the center frequency, or BEF. (C) Effects of auditory filtering on ambient noise. Upper trace is the roex model of auditory tuning for the Stengl Ranch population. Gray and black plots are the ambient noise spectra at Stengl Ranch (1-s sample) before and after passing through the model auditory filter. For this sample, the noise power in the auditory filter was 16.4 dB down from the field recording.
Roex filter parameters
. | Center frequency (Hz) . | ERB (Hz) . | Low slope (p) . | High slope (p) . | R2 . | p . |
|---|---|---|---|---|---|---|
| Gill | 3337 | 1634 | 7.042 | 9.051 | .959 | .0035 |
| Stengl | 3718 | 1613 | 8.69 | 9.817 | .927 | .0085 |
. | Center frequency (Hz) . | ERB (Hz) . | Low slope (p) . | High slope (p) . | R2 . | p . |
|---|---|---|---|---|---|---|
| Gill | 3337 | 1634 | 7.042 | 9.051 | .959 | .0035 |
| Stengl | 3718 | 1613 | 8.69 | 9.817 | .927 | .0085 |
Each column is the center frequency, equivalent rectangular bandwidth (ERB), low- and high-frequency slope parameters, and R2 and p values for the correlation of the roex model to each population's tuning curve.
Roex filter parameters
. | Center frequency (Hz) . | ERB (Hz) . | Low slope (p) . | High slope (p) . | R2 . | p . |
|---|---|---|---|---|---|---|
| Gill | 3337 | 1634 | 7.042 | 9.051 | .959 | .0035 |
| Stengl | 3718 | 1613 | 8.69 | 9.817 | .927 | .0085 |
. | Center frequency (Hz) . | ERB (Hz) . | Low slope (p) . | High slope (p) . | R2 . | p . |
|---|---|---|---|---|---|---|
| Gill | 3337 | 1634 | 7.042 | 9.051 | .959 | .0035 |
| Stengl | 3718 | 1613 | 8.69 | 9.817 | .927 | .0085 |
Each column is the center frequency, equivalent rectangular bandwidth (ERB), low- and high-frequency slope parameters, and R2 and p values for the correlation of the roex model to each population's tuning curve.
Assessing performance of the Gill and Stengl filters
To calculate the extent to which the tuning curves filtered ambient noise, the roex filters were applied to the power spectra of sixteen 1-s noise samples recorded on different nights from 16 consecutive weeks starting at the first week in May, between 1900 and 2400 h, at each location (N = 32; although frogs may be present earlier or later in the year, these months represent the main period of the cricket frog breeding season at these sites). Each spectral component of the noise was then adjusted by the corresponding filter value. Due to the number of samples in each 1-s recording (15,625-Hz sampling rate), the spectra were calculated using a complex Fourier transform (cfft, Mathcad); the spectra had a 1-Hz resolution and a bandwidth of 7812 Hz. The difference between the total power of the unfiltered and filtered ambient noise represents the amount of noise removed by the auditory filter (i.e., independent of absolute noise level). See Figure 1C for a graphic example of the filtering. This value was then copied into an Excel spreadsheet, and separate paired t tests (two tailed) were used to compare the noise-filtering performance of the Gill and Stengl filters with noise at each of the two habitats.
RESULTS
Noise in the two habitats
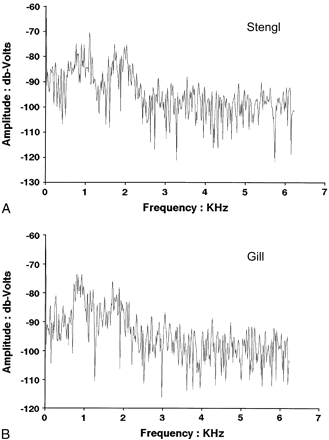
Example noise spectra from each habitat are shown in Figure 2. In both habitats, most environmental noise was between approximately 1500 and 2200 Hz. Sources of the noise were not characterized, but examination of the tapes indicated both abiotic and biotic (including insects and other anuran species) contributions. At some point between 2000 and 2500 Hz, energy in the noise declined and remained at a relatively steady level from approximately 2500 to 6000 Hz (the upper limit of our sampling). In the case of both the Gill and Stengl populations, both the average male call and the average female tuning would be centered above 3000 Hz, above the elevated noise plateaus in either population. In the field we had the impression that the background noise at Stengl Ranch was louder and contained more different sources of noise than the ambient noise at Gill Ranch. We did not exhaustively measure noise amplitude at various locations and times at each site, however, and therefore cannot compare noise amplitudes statistically with any validity. We note that all analyses, including the filtering comparisons, were performed in the frequency domain using relative amplitudes and therefore are independent of absolute intensity.
Example noise spectra from Stengl (A) and Gill (B) locations. Each is the FFT of a single 1-s sample.
Ambient noise throughout the season
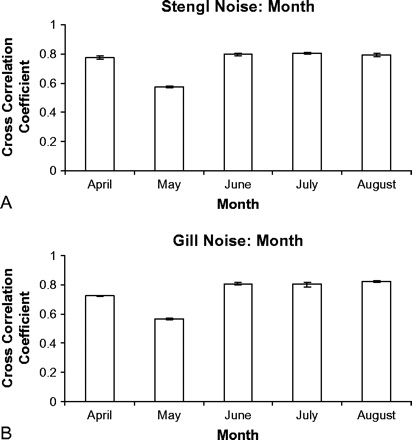
We determined if the noise changed throughout the reproductive season of cricket frogs between March and August (Figure 3). The mean cross-correlation coefficients at Stengl Ranch varied between 0.573 and 0.806 across months. There was a significant difference between the cross-correlation coefficients at different months (Kruskal-Wallis test, χ2 = 33.160, df = 4, n = 13 each month, p < .001). Inspection of the data suggests that this is due to the low mean coefficient in May, as the remaining coefficients are all between 0.776 and 0.806. At Gill Ranch the mean cross-correlation coefficients varied between 0.566 and 0.822 and again there was a significant difference between the cross-correlation coefficients at different months (Kruskal-Wallis test, χ2 = 29.785, df = 4, n = 14 each month, p < .001). As for the Stengl site, this seems likely due to the low mean correlation coefficient in May. We have not investigated the reason for the difference in noise quality in either case but suspect that it may be due to differences in insect noise. The mean monthly cross-correlation coefficients were not different for the Stengl and Gill recordings (t = 0.09, df = 8, p = .934, two tailed). Thus, at both sites the noise varied across the season, largely due to the influence of 1 month, but the two sites were similar in the extent of that variation.
Mean (±SE) cross-correlation coefficients of noise recorded each month from April to August (relative to noise recorded in March) at the Stengl (A) and Gill (B) locations.
Ambient noise at different times each night
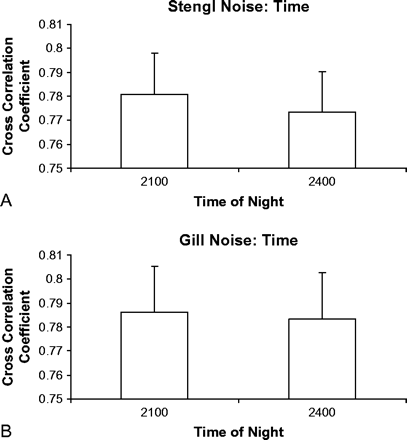
We examined variation in noise spectra throughout a night at each site (Figure 4). At Stengl Ranch we found no significant difference between the cross-correlation coefficient of noise recorded at 2100 h and the coefficients of noise recorded at 2400 h (Mann-Whitney U test, nl = n2 = 24, z = −0.361, p = .718). Similarly, there was no significant difference between noise cross-correlation coefficients for 2100 h versus 2400 h at the Gill Ranch site (Mann-Whitney U test, n1 = n2= 23, z = −1.604, p = .109). The mean cross-correlation coefficients of the Stengl noise were not significantly different than those of the Gill noise (t = 1.838, df = 2, p = .21, two tailed). Thus, the quality of the noise (measured by the FFT) was consistent throughout the night at both locations.
Mean (±SE) cross-correlation coefficients of noise recorded at 2100 and 2400 h (relative to noise recorded at 1800 h) at the Stengl (A) and Gill (B) locations.
Filter function performance
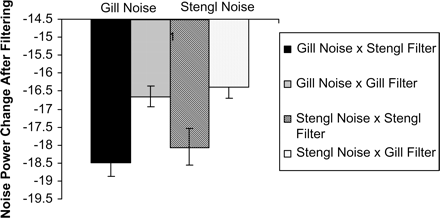
We investigated how much energy remained when the ambient noise from both locations was filtered with the Gill filter and Stengl filter at both places. Figure 5 shows the changes in power when the average noise from each habitat was filtered with each of the roex filter functions based on the female tuning curves. On average, for noise from both habitats, less noise remained after filtering with the Stengl filter, indicating that it was better than the Gill filter in filtering environmental noise. A paired t test (two tailed, 16 pairs) matching the two filters against the samples of ambient noise from the Gill Ranch habitat indicated a significant difference in filtering capabilities, with less noise remaining after passing through the Stengl filter (t = 16.28, df = 15, p << .001). A similar result was obtained using the ambient noise from the Stengl Ranch habitat (t = 7.74, df = 15, p << .001).
Mean (±SE) change in power (in arbitrary dB units) in the noise spectra from the Gill and Stengl locations after filtering with the Stengl and Gill filters. Larger negative numbers indicate less noise remaining thus indicating that more noise was rejected by the filter. Filtering is independent of absolute noise level.
We further examined whether either filter performed better in filtering noise from its own habitat than from the other habitat. For the Gill filter, there was no significant difference in the noise remaining after filtering ambient noise from Gill Ranch versus ambient noise from Stengl Ranch (t = −0.61, df = 30, p = .54, two tailed). Similarly, for the Stengl filter, there was no significant difference in the noise remaining after filtering ambient noise from Gill Ranch versus ambient noise from Stengl Ranch (t = −0.67, df = 30, p = .50, two tailed).
In sum, the results of the filter analysis indicate that the average female Stengl filter is better than the average female Gill filter in filtering ambient noise from both habitats. There is no indication that the filtering advantage is greater in either of the two habitats.
DISCUSSION
Our previous studies of A. crepitans showed that different populations of cricket frogs have significantly different BP tuning (Keddy-Hector et al., 1992; Ryan and Wilczynski, 1988; Wilczynski and Ryan, 1999; Wilczynski et al., 1992). Populations in forest habitats generally are tuned to higher frequencies than populations in open grassland habitats. That is the case for the two populations modeled here, where the Stengl population occupies a pine forest habitat and the Gill population resides in a more open grassland area. Stengl calls have a mean dominant frequency of 3820 Hz, while Gill calls average 3556 Hz; in both cases, call energy falls off gradually with higher and lower frequency such that there is little remaining energy less than 2000 Hz or more than 4500 Hz (Ryan and Wilczynski, 1991; Wilczynski and Ryan, 1999). This tuning variation preserves a general match between the spectral peak in the call and the peak sensitivity of the auditory system within populations. The traditional, and certainly valid, view has focused on this call-tuning relationship, with any differences in tuning characteristics regarded as being related to matching call features to aid reception (reviewed in Gerhardt and Schwartz, 2001). The results of this study suggest another, heretofore unappreciated, perspective on tuning differences, namely that they may in part reflect the results of habitat selection to limit noise interference.
An inspection of the noise spectra from both locations indicates that they are relatively similar in the two habitats, with most environmental noise concentrated in frequencies less than 2200 Hz followed by a relatively flat plateau from 2500 to 6000 Hz. Furthermore, the analyses of noise spectra over the cricket frog breeding season and at different times during a night show that there are few drastic shifts in noise characteristics in either case in either habitat, although there are some noise differences, as would be expected given the variable nature of environmental noise. The relative consistency in noise characteristics during a night and over the breeding season shows that cricket frogs in each of these two populations are exposed to a largely stable acoustical environment. Such an environment would provide the consistent selection regime that would be necessary to support the evolution of tuning shifts. We do note that our analysis is restricted to one population in each habitat type, and therefore we cannot conclusively show that this is a general phenomenon throughout the cricket frog range.
It may be that absolute noise level changes over a night or season. We did not measure seasonal or nightly variations in level. Furthermore, our tests of the filter functions did not take intensity differences into account, as we assumed that the filter functions' location in frequency space should more closely reflect responses to the composition, rather than the amplitude, of environmental noise, which would vary greatly among individual positions within a chorus. Although we do not have measurements of absolute noise levels, our impression is that average noise level is higher in the Stengl habitat than in the Gill habitat. For firm conclusions to be drawn, this would have to be confirmed with noise amplitude measurements made with the same systematic regime applied to the noise spectra recordings. Nevertheless, if we accept that our measurements show that the spectral profile of ambient noise is approximately the same in the two habitats, we can speculate on the basis for the observed habitat differences in auditory filtering.
Our analysis of auditory filtering is based on modeling the BP-filtering characteristics of an average female from each of two populations that reside in the different habitats Stengl, characterized by denser pine forest vegetation, and Gill, which is a more open grassland habitat. The analysis essentially asks, how would an average female from each population fare in its ability to filter environmental noise? The results show that the average auditory filter from the Stengl population performed better at filtering environmental noise than the filter from the Gill population. The Stengl filter performs better in both habitats. Therefore, we can reject the hypothesis that each filter is optimized for performance in its own habitat. Rather, the results support the hypothesis that one filter, the Stengl filter, is absolutely better in filtering environmental noise and therefore manifests this when confronted with noise from either habitat. Given the similarity we found in the general shape of the noise spectra in the two habitats, it would be expected that a filter that performed better in one habitat would also perform better in the other. The analysis was performed using noise samples randomly selected from different times of night and days throughout the breeding season, and, moreover, the noise analysis showed that the spectral content of the noise was relatively stable throughout the season. For these reasons, we believe that the effects we demonstrated are not spurious results due to sampling noise at peculiar times.
We found a similar result in previous work on transmission characteristics of the male calls from these populations (Ryan et al., 1991). The average Stengl male call transmits with less degradation in both the Stengl and Gill habitats. That earlier study (Ryan et al., 1991) further indicated that the pine forest Stengl habitat was far more acoustically challenging than the open grassland of the Gill habitat, where neither call degraded to an appreciable degree over the same several meter distances that caused severe degradation in the Stengl habitat. A recent study of microhabitat acoustics in both habitat types (Venator, 1999) showed that calls both degraded and attenuated more precipitously with distance in the pine forest.
The greater degradation and attenuation of the call in acoustically cluttered habitats like the pine forest of the Stengl site and, possibly, greater noise amplitudes suggested by our subjective impressions at that site may be the main factors contributing to the selection for better filtering in the Stengl frogs rather than the differences in the spectral composition of the noise, which our data suggest are minimal. Both increased noise levels and increased signal attenuation and degradation during transmission would exacerbate the problem of detecting a signal in a noisy environment. Filtering out more noise would improve the signal-to-noise ratio, thus enhancing the ability to detect and recognize the call.
It should be noted that there are several other important considerations for understanding signal-noise-filter interactions in different habitats. Cricket frog calls (the signal) change spectrally as well as temporally and in amplitude during transmission, and these changes depend on microenvironment even within a particular habitat (Venator, 1999; Sun et al., 2000). An earlier modeling study (Sun et al., 2000) showed that receiver performance in capturing the signal decreased with these distance-related changes. Because the transmission effects are greater in the pine forest habitat, receiver performance would be degraded even more there, increasing the pressure to improve detection by decreasing noise interference. In addition, high ambient noise can increase the threshold of auditory fibers (the receiver), which may change its relative sensitivity to both signals and ambient noise (Narins, 1987). It is not clear how this would change the signal-to-noise ratio during an actual acoustic interaction. These considerations will be different for each individual in a chorus, however, and we cannot incorporate them into our model at this time because we cannot specify the positions and amplitudes of all senders and noise sources relative to each receiver, nor the microenvironmental transmission paths among all of them. Furthermore, changes in the signal as a result of its spectral composition being altered by the peripheral filter along with the noise is an important factor in how well an individual is ultimately able detect a call. The signal-to-noise ratio after filtering compared to before filtering has occurred is the true determinant of whether the filtering helps call detection. A statistical test of this will have to await a more extensive modeling effort following the one described here. Despite all these complications, we believe our results are valid in indicating how an average female would filter out extraneous ambient noise encountered during a typical breeding season.
Taken together, the previous results on call transmission and the present study on receiver filtering from these two populations suggest that both parts of the communication system in this species, the sender (the call) and the receiver (the peripheral auditory system) are different in the forest population in order to compensate for the problems induced by the more challenging habitat acoustics there. The call has changed its temporal characteristics to allow it to transmit with less degradation, and the peripheral auditory system has changed its filtering properties to reduce the effects of environmental noise and so reduce masking of the more attenuated signal there. Grassland habitats of the type occupied by the Gill population represent far fewer challenges in this regard (Marten and Marler, 1977; Wiley and Richards, 1982). As a consequence, both the average male call and the average female auditory filtering of Stengl frogs perform better than those of the Gill frogs, and this advantage is apparent in the native Stengl habitat as well as the grassland habitats of the other population.
In principle, a receiver could evolve changes in its absolute sensitivity and in its filter properties. Varying the boundaries of a filter function will change the range of noise that a receiver will detect, either by shifting the boundaries of that function to avoid troublesome stimuli or narrowing its bandwidth to decrease the range of potentially interfering signals. The two cricket frog populations differ in both features, with the Stengl filter shifted slightly farther from the major peaks of the noise spectra and slightly narrowed in bandwidth. It is possible that varying one feature passively alters the other due to some mechanical constraint involving the ear. This would complicate the interpretation of the evolution of this system. We cannot investigate this experimentally, and comparisons across species are problematic. However, reexamining our previously published single-unit data from cricket frogs (Keddy-Hector et al., 1992; Wilczynski et al., 1992) suggests that changes in best frequency and bandwidth are not necessarily linked. Across five cricket frog populations from which we have good single-unit measures of VIII nerve tuning and Q10 values, there is a trend for populations with high BEFs to have higher Q10 values (i.e., narrower tuning; Pearson r = .75, N= 5, p = .146). However, the data are confounded by the fact that the higher and more narrowly tuned populations are all from pine forest habitats, two of which may represent a separate subspecies. More persuasive is the correlation analysis of Stengl population females, where we have clear tuning data from 10 individuals. Here, without confounding animals from different habitats or subspecies, we find no evidence for correlated changes in BEF and Q10 (Pearsons r = −.009, N = 10, p = .981).
There is no evidence from our neurophysiological data that cricket frog populations vary in the other possible change in their filter functions, their absolute thresholds (Venator, 1999). It is not clear that such a change would really be beneficial in the actual noisy environments in which frogs communicate, as high noise levels would mask any low-amplitude signal that would potentially be detected by a highly sensitive auditory system.
Examining the environmental factors underlying variation in acoustic communication signals has had a long history, beginning with Morton's (1975) seminal studies on birds. These studies have provided evidence for environmental influences on vocal signals or on vocalization behavior in birds (Bowman, 1983; Ryan and Brenowitz, 1985; Slabbekoorn and Smith, 2002; Wiley and Richards, 1982), frogs (Lardner and bin Lakim, 2002; Penna and Solis, 1996; Ryan et al., 1991), insects (Bailey et al., 2001), and primates (Brumm et al., 2004). In contrast, the receiver has been largely neglected other than to assess how call differences relate to differences in sensory coding. Our results indicate that the acoustic environment may also influence the evolution of auditory tuning. In cricket frogs, evidence suggests that environmental selection imposed by the more acoustically challenging pine forest habitat has made an impact on both parts of the communication dyad. In the environment in which frog calls attenuate and degrade faster over distance (Ryan et al., 1991; Venator, 1999), populations have evolved calls that transmit with less attenuation and better fidelity than cricket frog calls from other types of habitats (Ryan et al., 1991). Our current results in these two populations show that the forest population has also evolved auditory filters that are better at filtering out environmental noise typical of the habitats in which cricket frogs live. The evidence that environmental acoustics may be a factor in the evolution of both signals and receivers suggests the need for considering the multiple interactions among three factors in guiding the evolution of acoustic communication systems, the calls of the receivers, the tuning of the auditory system, and the composition and level of environmental noise, and it highlights the challenging task of understanding the cause and effect relationships among them that help generate observed patterns of evolution.
K. Witte is now at the Lehrstuhl für Verhaltensforschung, Universität Bielefeld, Postfach 100131, 33615 Bielefeld, Germany. H.E. Farris is now at the Center for Neuroscience, Louisiana State University, Health Science Center, 2020 Gravier Street, New Orleans, LA 70112, USA.
This work was supported by DFG Wi 1531 to K.W. and NIMH RO1 MH52696 to W.W. and M.J.R.
References
Bailey WJ, Bennet-Clarke HC, Fletcher NH,
Boughman JW,
Bowman RI,
Brenowitz EA,
Brumm H, Voss K, Koellmer I, Todt D,
Burmeister S, Konieczka J, Wilczynski W,
Burmeister S, Ophir AG, Ryan MJ, Wilczynski W,
Burmeister S, Wilczynski W, Ryan MJ,
Capranica RR, Moffat AJM,
Cummings ME, Partridge JC,
Ehret G, Capranica RR,
Endler JA,
Endler JA,
Endler JA,
Farris HE, Hoy RR,
Finneran JJ, Schlundt CE, Carter DA, Ridgeway SH,
Fleishmann LJ,
Gerhardt HC, Klump GM,
Gerhardt HC, Schwartz JJ,
Gish SL, Morton ES,
Keddy-Hector AC, Wilczynski W, Ryan MJ,
Lall AB, Seliger HH, Biggley WH, Lloyd JE,
Langemann U, Gauger B, Klump GM,
Lythgoe JN, Muntz WRA, Partridge JC, Shand J, Williams DMcB,
Marchetti K,
Marten K, Marler P,
Moore BCJ, Peters RW, Glasberg BR,
Narins PM,
Narins PM,
Nevo E, Capranica RR,
Patterson RD, Nimmo-Smith I, Weber DL, Milroy R.,
Penna M, Solis R,
Perrill SA, Shepherd WJ,
Reimchen TE,
Richards DG, Wiley RH,
Ryan MJ, Brenowitz EA,
Ryan MJ, Cocroft RB, Wilczynski W,
Ryan MJ, Kime NM,
Ryan MJ, Perrill SA, Wilczynski W,
Ryan MJ, Sullivan BK,
Ryan MJ, Wilczynski W,
Ryan MJ, Wilczynski W,
Slabbekoorn H, Smith TB,
Sun L-X, Wilczynski W, Rand AS, Ryan MJ,
Venator KR,
Wagner WE Jr,
Waser PM, Waser MS,
Wilczynski W, Keddy-Hector AC, Ryan MJ,
Wilczynski W, Ryan MJ,
Wiley RH, Richards DG,
Wiley RH, Richards DG,
Witte K, Ryan MJ, Wilczynski W,
Wollerman L, Wiley H,
Author notes
aSection of Integrative Biology, University of Texas, Austin, TX 78712, USA
bDepartment of Psychology, University of Texas, 1 University Station A8000, Austin, TX 78712-0187, USA